Allogeneic hematopoietic stem-cell transplantation for sickle cell disease - PubMed (original) (raw)
Clinical Trial
Allogeneic hematopoietic stem-cell transplantation for sickle cell disease
Matthew M Hsieh et al. N Engl J Med. 2009.
Abstract
Background: Myeloablative allogeneic hematopoietic stem-cell transplantation is curative in children with sickle cell disease, but in adults the procedure is unduly toxic. Graft rejection and graft-versus-host disease (GVHD) are additional barriers to its success. We performed nonmyeloablative stem-cell transplantation in adults with sickle cell disease.
Methods: Ten adults (age range, 16 to 45 years) with severe sickle cell disease underwent nonmyeloablative transplantation with CD34+ peripheral-blood stem cells, mobilized by granulocyte colony-stimulating factor (G-CSF), which were obtained from HLA-matched siblings. The patients received 300 cGy of total-body irradiation plus alemtuzumab before transplantation, and sirolimus was administered afterward.
Results: All 10 patients were alive at a median follow-up of 30 months after transplantation (range, 15 to 54). Nine patients had long-term, stable donor lymphohematopoietic engraftment at levels that sufficed to reverse the sickle cell disease phenotype. Mean (+/-SE) donor-recipient chimerism for T cells (CD3+) and myeloid cells (CD14+15+) was 53.3+/-8.6% and 83.3+/-10.3%, respectively, in the nine patients whose grafts were successful. Hemoglobin values before transplantation and at the last follow-up assessment were 9.0+/-0.3 and 12.6+/-0.5 g per deciliter, respectively. Serious adverse events included the narcotic-withdrawal syndrome and sirolimus-associated pneumonitis and arthralgia. Neither acute nor chronic GVHD developed in any patient.
Conclusions: A protocol for nonmyeloablative allogeneic hematopoietic stem-cell transplantation that includes total-body irradiation and treatment with alemtuzumab and sirolimus can achieve stable, mixed donor-recipient chimerism and reverse the sickle cell phenotype. (ClinicalTrials.gov number, NCT00061568.)
2009 Massachusetts Medical Society
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
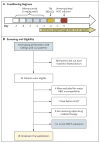
Figure 1. Conditioning Regimen and Screening of Patients
Panel A shows the conditioning regimen used before hematopoietic stem-cell transplantation (HSCT). The regimen consisted of alemtuzumab, at a total dose of 1 mg per kilogram of body weight, given over a period of 5 days in gradually increasing doses: 0.03 mg per kilogram (test dose) on day −7, 0.1 mg per kilogram on day −6, and then 0.3 mg per kilogram per day on days −5 through −3. A single dose of 300 cGy of total-body irradiation (TBI) was administered on day −2 (with gonadal shielding applied for men). Treatment with oral sirolimus was initiated on day −1 at a dose of 5 mg every 4 hours for three doses, then 5 mg daily starting on day 0, modified to achieve target trough levels of 10 to 15 ng per milliliter of whole blood. Panel B shows the numbers of patients and their siblings who were screened, the number of eligible patients, and the number of patients who underwent transplantation. Among a total of 24 eligible patients, 6 of 6 HLA-matched siblings were identified. Four patients were excluded owing to major ABO incompatibility and a previously reported high incidence of pure red-cell aplasia from residual recipient lymphoid cells in nonmyeloablative HSCT,, and one patient died of suspected sudden arrhythmia from severe iron overload 1 month before the planned HSCT. Eight patients are currently undergoing optimization of their medical therapy, including hydroxyurea therapy, iron chelation, and pain management, and one patient is currently undergoing evaluation for HSCT.
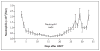
Figure 2. Neutrophil Counts after Hematopoietic Stem-Cell Transplantation (HSCT)
Each circle represents the mean neutrophil count for all patients after the transplantation procedure. I bars indicate standard errors.
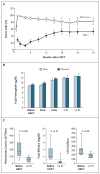
Figure 3. Donor-Cell Chimerism and Laboratory Measurements after Hematopoietic Stem-Cell Transplantation
Panel A shows the mean (±SE) percentage of donor cells after hematopoietic stem-cell transplantation (HSCT). Donor chimerism among CD3+ T cells and CD14+15+ myeloid cells was assessed by means of a polymerase-chain-reaction assay to determine minisatellite polymorphisms between patient and donor. Panel B shows the laboratory measurements obtained one month before HSCT and those obtained at the most recent follow-up (median, 30 months; range, 15 to 54). The bar graphs in Panel B show mean values for total hemoglobin before, during (Exch), and after HSCT for male and female recipients; T bars indicate standard errors. For the reticulocyte count, total bilirubin level, and lactate dehydrogenase (LDH) level shown in Panel C, the horizontal lines within the box plots indicate the mean values, the lower and upper ends of the boxes represent the 25th and 75th percentiles, and the I bars represent the 10th and 90th percentiles; the dashed lines represent the upper limits of the reference ranges.
Comment in
- Hematopoietic stem-cell transplantation for adults with sickle cell disease.
Abboud MR. Abboud MR. N Engl J Med. 2009 Dec 10;361(24):2380-1. doi: 10.1056/NEJMe0908574. N Engl J Med. 2009. PMID: 20007564 No abstract available. - Stem-cell transplantation for sickle cell disease.
Dew A, van Besien K. Dew A, et al. N Engl J Med. 2010 Mar 11;362(10):955; author reply 956. doi: 10.1056/NEJMc1000134. N Engl J Med. 2010. PMID: 20220194 No abstract available. - Stem-cell transplantation for sickle cell disease.
Walters MC, Sullivan KM. Walters MC, et al. N Engl J Med. 2010 Mar 11;362(10):955-6; author reply 956. N Engl J Med. 2010. PMID: 20225347 No abstract available.
Similar articles
- Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype.
Hsieh MM, Fitzhugh CD, Weitzel RP, Link ME, Coles WA, Zhao X, Rodgers GP, Powell JD, Tisdale JF. Hsieh MM, et al. JAMA. 2014 Jul 2;312(1):48-56. doi: 10.1001/jama.2014.7192. JAMA. 2014. PMID: 25058217 Free PMC article. Clinical Trial. - Azathioprine/hydroxyurea preconditioning prior to nonmyeloablative matched sibling donor hematopoietic stem cell transplantation in adults with sickle cell disease: A prospective observational cohort study.
Dovern E, Aydin M, Hazenberg MD, Tang MW, Suijk EM, Hoogendoorn GM, Van Tuijn CFJ, Kerkhoffs JL, Rutten CE, Zeerleder SS, de la Fuente J, Biemond BJ, Nur E. Dovern E, et al. Am J Hematol. 2024 Aug;99(8):1523-1531. doi: 10.1002/ajh.27360. Epub 2024 May 11. Am J Hematol. 2024. PMID: 38733340 - Nonmyeloablative Stem Cell Transplantation with Alemtuzumab/Low-Dose Irradiation to Cure and Improve the Quality of Life of Adults with Sickle Cell Disease.
Saraf SL, Oh AL, Patel PR, Jalundhwala Y, Sweiss K, Koshy M, Campbell-Lee S, Gowhari M, Hassan J, Peace D, Quigley JG, Khan I, Molokie RE, Hsu LL, Mahmud N, Levinson DJ, Pickard AS, Garcia JG, Gordeuk VR, Rondelli D. Saraf SL, et al. Biol Blood Marrow Transplant. 2016 Mar;22(3):441-8. doi: 10.1016/j.bbmt.2015.08.036. Epub 2015 Sep 5. Biol Blood Marrow Transplant. 2016. PMID: 26348889 Clinical Trial. - Curative therapies: Allogeneic hematopoietic cell transplantation from matched related donors using myeloablative, reduced intensity, and nonmyeloablative conditioning in sickle cell disease.
Guilcher GMT, Truong TH, Saraf SL, Joseph JJ, Rondelli D, Hsieh MM. Guilcher GMT, et al. Semin Hematol. 2018 Apr;55(2):87-93. doi: 10.1053/j.seminhematol.2018.04.011. Epub 2018 Apr 25. Semin Hematol. 2018. PMID: 29958564 Free PMC article. Review.
Cited by
- Hematopoietic Stem Cell Transplantation in Adult Sickle Cell Disease: Problems and Solutions.
Özdoğu H, Boğa C. Özdoğu H, et al. Turk J Haematol. 2015 Sep;32(3):195-205. doi: 10.4274/tjh.2014.0311. Turk J Haematol. 2015. PMID: 25912490 Free PMC article. Review. - The role of donor-derived veto cells in nonmyeloablative haploidentical HSCT.
Or-Geva N, Reisner Y. Or-Geva N, et al. Bone Marrow Transplant. 2015 Jun;50 Suppl 2:S14-20. doi: 10.1038/bmt.2015.89. Bone Marrow Transplant. 2015. PMID: 26039201 - Sickle cell disease in children.
Meier ER, Miller JL. Meier ER, et al. Drugs. 2012 May 7;72(7):895-906. doi: 10.2165/11632890-000000000-00000. Drugs. 2012. PMID: 22519940 Free PMC article. Review. - Rapid complete donor lymphoid chimerism and graft-versus-leukemia effect are important in early control of chronic lymphocytic leukemia.
Shaffer BC, Modric M, Stetler-Stevenson M, Arthur DC, Steinberg SM, Liewehr DJ, Fowler DH, Gale RP, Bishop MR, Pavletic SZ. Shaffer BC, et al. Exp Hematol. 2013 Sep;41(9):772-8. doi: 10.1016/j.exphem.2013.04.015. Epub 2013 May 18. Exp Hematol. 2013. PMID: 23689118 Free PMC article. Clinical Trial. - Pharmacologic modulation of niche accessibility via tyrosine kinase inhibition enhances marrow and thymic engraftment after hematopoietic stem cell transplantation.
Fewkes NM, Krauss AC, Guimond M, Meadors JL, Dobre S, Mackall CL. Fewkes NM, et al. Blood. 2010 May 20;115(20):4120-9. doi: 10.1182/blood-2009-10-248898. Epub 2010 Mar 15. Blood. 2010. PMID: 20231424 Free PMC article.
References
- Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110:543–8. - PubMed
- Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180:326–8. - PubMed
- Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–76. - PubMed
- Vermylen C, Cornu G, Ferster A, et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant. 1998;22:1–6. - PubMed
- Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials