Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle - PubMed (original) (raw)
Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle
Eri Ishikawa et al. J Exp Med. 2009.
Abstract
Tuberculosis remains a fatal disease caused by Mycobacterium tuberculosis, which contains various unique components that affect the host immune system. Trehalose-6,6'-dimycolate (TDM; also called cord factor) is a mycobacterial cell wall glycolipid that is the most studied immunostimulatory component of M. tuberculosis. Despite five decades of research on TDM, its host receptor has not been clearly identified. Here, we demonstrate that macrophage inducible C-type lectin (Mincle) is an essential receptor for TDM. Heat-killed mycobacteria activated Mincle-expressing cells, but the activity was lost upon delipidation of the bacteria; analysis of the lipid extracts identified TDM as a Mincle ligand. TDM activated macrophages to produce inflammatory cytokines and nitric oxide, which are completely suppressed in Mincle-deficient macrophages. In vivo TDM administration induced a robust elevation of inflammatory cytokines in sera and characteristic lung inflammation, such as granuloma formation. However, no TDM-induced lung granuloma was formed in Mincle-deficient mice. Whole mycobacteria were able to activate macrophages even in MyD88-deficient background, but the activation was significantly diminished in Mincle/MyD88 double-deficient macrophages. These results demonstrate that Mincle is an essential receptor for the mycobacterial glycolipid, TDM.
Figures
Figure 1.
Mincle recognizes mycobacterial species. (A–C) NFAT-GFP reporter cells expressing FcRγ only (FcRγ), Mincle + FcRγ, and MincleEPN→QPD + FcRγ were co-cultured for 18 h with heat-killed M. smegmatis (A), M. bovis (B), or M. tuberculosis H37 Rv (C). Induction of NFAT-GFP was analyzed by flow cytometry. (D) Reporter cells expressing Dectin-2 + FcRγ were stimulated with M. smegmatis, M. bovis, and Candida albicans (C. a.). (E) Reporter cells expressing Mincle + FcRγ were stimulated with 107 M. smegmatis together with anti-Mincle mAb and rat IgG1 as a control. (F) Cells were stimulated with wild-type M. smegmatis (WT), pimE-deficient M. smegmatis (Δ_pimE), and pimE-deficient M. smegmatis reconstituted with pimE (Δ_pimE + pimE). All data are means ± SD for triplicate assays, and representative results from three independent experiments with similar results are shown.
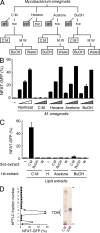
Figure 2.
Mincle recognizes mycobacterial trehalose-6,6′-dimycolate. (A) Schematic diagram of delipidation of M. smegmatis. Delipidated bacteria (gray boxes) and lipid extracts (open boxes) were applied for ligand assays in B and C, respectively. (B and C) Heat-killed M. smegmatis treated with C:M, hexane, acetone, or 1-butanol (BuOH; B), and plate-coated lipid extract (C) were co-cultured with reporter cells expressing Mincle + FcRγ. (D) C:M phase of C:M extract was analyzed by HPTLC and divided into 22 subfractions. Each subfraction was coated onto a plate to stimulate reporter cells. Purified TDM was used as a reference (right lane). Arrowheads show the origin and solvent front. Data are means ± SD for triplicate assays (B and C) or means for duplicate assays (D). Representative results from three independent experiments with similar results are shown.
Figure 3.
Purified TDM is recognized by Mincle. (A) Chemical structure of TDM. α-Mycolate (shown), methoxy-mycolate, and keto-mycolate are the major subclasses of mycolate found in M. tuberculosis TDM. (B and C) Reporter cells were stimulated with the indicated amount of plate-coated TDM (B) or TDB (C). (D) Reporter cells were stimulated with the indicated amount of TDM, methyl α-mycolate (α-mycolate), or methyl keto-mycolate (keto-mycolate). (E) Reporter cells were stimulated with the indicated amount of TDM and TMM. (F) ELISA-based detection of TDM by Mincle-Ig. hIgG1-Fc (Ig), Mincle-Ig, and Dectin2-Ig were incubated with 0.1 nmol/0.32 cm2 of plate-coated TDM. Bound protein was detected with anti–hIgG-HRP followed by the addition of colorimetric substrate. (G) Effect of trehalose (100 µg/ml), EDTA (10 mM), rat IgG (10 µg/ml), and anti-Mincle mAb (10 µg/ml) on TDM recognition by Mincle-Ig. ELISA-based detection was performed as in E. (H) Reporter cells were stimulated with TDM, which was treated with trehalase as described in Materials and methods. Cells were also stimulated with plate-coated anti-Mincle mAb treated with trehalase as a negative control. All data are means ± SD for triplicate assays and representative results from three independent experiments with similar results are shown.
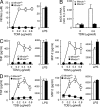
Figure 4.
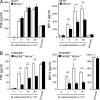
Lack of TDM-mediated activation in Mincle-deficient macrophages. (A) BMMφ from Mincle+/− and Mincle−/− were primed with IFN-γ (10 ng/ml) and stimulated with plate-coated TDM or LPS (10 ng/ml) as a control. Culture supernatants were collected at 48 h and concentration of NO was measured. (B) IFN-γ–primed BMMφ were stimulated with plate-coated TDM for 36 h as in A, and mRNA expression of iNOS was analyzed by real-time PCR. (C and D) IFN-γ–primed BMMφ were stimulated with plate-coated TDM (C) or TDB (D) for 24 h. Culture supernatants were collected and concentrations of TNF and MIP-2 were determined by ELISA. All data are means ± SD for triplicate assays and representative results from three independent experiments with similar results are shown.
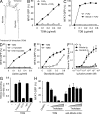
Figure 5.
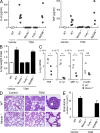
Mincle is essential for TDM-induced inflammation in vivo. (A) Mincle+/+, Mincle−/−, FcRγ−/− and Myd88−/− mice were injected intravenously with an oil-in-water emulsion containing TDM (150 µg). Emulsion without TDM was injected as a vehicle control. At day 1 after injection, IL-6 and TNF concentrations in sera were determined by ELISA. Each symbol represents an individual mouse. (B) Lungs of TDM-injected mice were removed at day 7 and inflammatory intensity was evaluated by calculation of LWI. (C) Proinflammatory mediator mRNA levels in lungs at day 7 after TDM administration were evaluated by real-time PCR. Relative expression levels are shown as 2−_C_t. Each symbol represents an individual mouse. Ct, cycle threshold. *, P < 0.05; **, P < 0.001. NS, not significant. (D) Histology of the lungs from untreated (control) and TDM-injected (TDM) mice was examined by hematoxylin-eosin staining at day 7. Bar, 0.1 mm. (E) Number of lung granulomas. Granuloma in lungs from mice injected with TDM was counted as described in Materials and methods. Data are means ± SD for the mean number for at least three independent mice. Representative results from two independent experiments with similar results are shown.
Figure 6.
Mincle is responsible for the TLR-independent pathway of anti-mycobacterium responses. (A and B) BMMφ from WT, Mincle−/− (A), MyD88−/−, and MyD88−/−Mincle−/− (B) mice were stimulated with indicated number of heat-killed M. tuberculosis H37Rv. Cells were also stimulated with zymosan as a positive control. At day 2 after stimulation, production of TNF and MIP-2 in supernatants was determined. Data are means ± SD for triplicate assays and representative results from three independent experiments with similar results are shown. *, P < 0.05; **, P < 0.01.
Similar articles
- Mincle is a long sought receptor for mycobacterial cord factor.
Matsunaga I, Moody DB. Matsunaga I, et al. J Exp Med. 2009 Dec 21;206(13):2865-8. doi: 10.1084/jem.20092533. Epub 2009 Dec 14. J Exp Med. 2009. PMID: 20008525 Free PMC article. - Differential control of Mincle-dependent cord factor recognition and macrophage responses by the transcription factors C/EBPβ and HIF1α.
Schoenen H, Huber A, Sonda N, Zimmermann S, Jantsch J, Lepenies B, Bronte V, Lang R. Schoenen H, et al. J Immunol. 2014 Oct 1;193(7):3664-75. doi: 10.4049/jimmunol.1301593. Epub 2014 Aug 25. J Immunol. 2014. PMID: 25156364 - Macrophage Phosphoproteome Analysis Reveals MINCLE-dependent and -independent Mycobacterial Cord Factor Signaling.
Hansen M, Peltier J, Killy B, Amin B, Bodendorfer B, Härtlova A, Uebel S, Bosmann M, Hofmann J, Büttner C, Ekici AB, Kuttke M, Franzyk H, Foged C, Beer-Hammer S, Schabbauer G, Trost M, Lang R. Hansen M, et al. Mol Cell Proteomics. 2019 Apr;18(4):669-685. doi: 10.1074/mcp.RA118.000929. Epub 2019 Jan 11. Mol Cell Proteomics. 2019. PMID: 30635358 Free PMC article. - Immune Recognition of Pathogen-Derived Glycolipids Through Mincle.
Miyake Y, Yamasaki S. Miyake Y, et al. Adv Exp Med Biol. 2020;1204:31-56. doi: 10.1007/978-981-15-1580-4_2. Adv Exp Med Biol. 2020. PMID: 32152942 Review. - Mincle: 20 years of a versatile sensor of insults.
Lu X, Nagata M, Yamasaki S. Lu X, et al. Int Immunol. 2018 May 24;30(6):233-239. doi: 10.1093/intimm/dxy028. Int Immunol. 2018. PMID: 29726997 Review.
Cited by
- Differential Host Pro-Inflammatory Response to Mycobacterial Cell Wall Lipids Regulated by the Mce1 Operon.
Petrilli JD, Müller I, Araújo LE, Cardoso TM, Carvalho LP, Barros BC, Teixeira M, Arruda S, Riley LW, Queiroz A. Petrilli JD, et al. Front Immunol. 2020 Aug 18;11:1848. doi: 10.3389/fimmu.2020.01848. eCollection 2020. Front Immunol. 2020. PMID: 32973761 Free PMC article. - HUMAN MICROBIOTA. Small molecules from the human microbiota.
Donia MS, Fischbach MA. Donia MS, et al. Science. 2015 Jul 24;349(6246):1254766. doi: 10.1126/science.1254766. Epub 2015 Jul 23. Science. 2015. PMID: 26206939 Free PMC article. Review. - Th17 cells in immunity to Candida albicans.
Hernández-Santos N, Gaffen SL. Hernández-Santos N, et al. Cell Host Microbe. 2012 May 17;11(5):425-35. doi: 10.1016/j.chom.2012.04.008. Cell Host Microbe. 2012. PMID: 22607796 Free PMC article. Review. - Formation and Maturation of the Phagosome: A Key Mechanism in Innate Immunity against Intracellular Bacterial Infection.
Lee HJ, Woo Y, Hahn TW, Jung YM, Jung YJ. Lee HJ, et al. Microorganisms. 2020 Aug 25;8(9):1298. doi: 10.3390/microorganisms8091298. Microorganisms. 2020. PMID: 32854338 Free PMC article. Review. - Deoxyfluoro-d-trehalose (FDTre) analogues as potential PET probes for imaging mycobacterial infection.
Rundell SR, Wagar ZL, Meints LM, Olson CD, O'Neill MK, Piligian BF, Poston AW, Hood RJ, Woodruff PJ, Swarts BM. Rundell SR, et al. Org Biomol Chem. 2016 Sep 28;14(36):8598-609. doi: 10.1039/c6ob01734g. Epub 2016 Aug 25. Org Biomol Chem. 2016. PMID: 27560008 Free PMC article.
References
- Billiau A., Matthys P. 2001. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 70:849–860 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases