Bordetella evades the host immune system by inducing IL-10 through a type III effector, BopN - PubMed (original) (raw)
Bordetella evades the host immune system by inducing IL-10 through a type III effector, BopN
Kanna Nagamatsu et al. J Exp Med. 2009.
Abstract
The inflammatory response is one of several host alert mechanisms that recruit neutrophils from the circulation to the area of infection. We demonstrate that Bordetella, a bacterial pathogen, exploits an antiinflammatory cytokine, interleukin-10 (IL-10), to evade the host immune system. We identified a Bordetella effector, BopN, that is translocated into the host cell via the type III secretion system, where it induces enhanced production of IL-10. Interestingly, the BopN effector translocates itself into the nucleus and is involved in the down-regulation of mitogen-activated protein kinases. Using pharmacological blockade, we demonstrated that BopN-induced IL-10 production is mediated, at least in part, by its ability to block the extracellular signal-regulated kinase pathway. We also showed that BopN blocks nuclear translocation of nuclear factor kappaB p65 (NF-kappaBp65) but, in contrast, promotes nuclear translocation of NF-kappaBp50. A BopN-deficient strain was unable to induce IL-10 production in mice, resulting in the elimination of bacteria via neutrophil infiltration into the pulmonary alveoli. Furthermore, IL-10-deficient mice effectively eliminated wild-type as well as BopN mutant bacteria. Thus, Bordetella exploits BopN as a stealth strategy to shut off the host inflammatory reaction. These results explain the ability of Bordetella species to avoid induction of the inflammatory response.
Figures
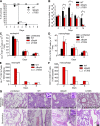
Figure 1.
BopN is involved in the up-regulation of IL-10. (A and B) DC2.4 cells were cultured in the absence (A) or presence (B) of 1 µM cytochalasin D (Cyto.D) for 30 min, and then infected with B. bronchiseptica WT, BopN effector mutant (ΔBopN), ΔBopN mutant complemented with a WT bopN clone (ΔBopN/pBopN), BopC effector mutant (ΔBopC), or T3SS mutant (ΔT3SS). After 60 min, total RNA was prepared and levels of IL-10 mRNA were assessed by real-time PCR using the comparative cycle threshold method. (C) BMDCs were infected with the indicated strains. After 60 min, total RNA was prepared and amounts of IL-10 mRNAs were assessed by real-time PCR. The values in A–C were normalized to the internal control β-actin and calculated in arbitrary units set to a value of 1 for uninfected cells. (D) BMDCs were infected with the indicated strains. After 60 min, the amount of IL-10 in the culture medium was determined by ELISA. The values are means ± SE from three independent experiments. *, P < 0.05.
Figure 2.
BopN is required for disease process. (A) C57BL/6J mice (n = 27 for each group) were infected intranasally with 5 × 106 B. bronchiseptica WT, ΔbopN, or ΔT3SS, and the survival was monitored for 8 d after infection. ***, P < 0.0001 using the log-rank test. (B) Mice (n = 15 for each group) infected intranasally with the specified strains (5 × 105 bacteria) were sacrificed at the indicated days after infection. Lung specimens were homogenized and plated on BG agar plates. Colonies were counted to determine the number of colonized bacteria per lung. The values are means ± SD from three independent experiments. *, P < 0.05. (C and D) Mice (n = 9 for each group) infected intranasally with the indicated strains (5 × 105 bacteria) were sacrificed, and cells were isolated from the whole lung. The total numbers of neutrophils (C) or macrophages (D) in the lung were determined by FACS analysis. The values are means ± SE from three independent experiments. *, P < 0.05. (E and F) The total number of neutrophils or macrophages in the lung obtained from C or D, respectively, was normalized by the bacterial number in the lung obtained from B. The values are means ± SE from three independent experiments. (G) 2 d after infection (5 × 105 bacteria), lung sections were fixed and stained with H&E. The infiltration of neutrophils and macrophages into the pulmonary alveoli and peribronchiolar edema formation are observed in mice infected with WT, ΔbopN, and ΔT3SS. Arrows indicate bronchioles. The number of neutrophils per 100 µm2 was determined in three randomly obtained images of pulmonary alveoli. The area of the enlarged boxes corresponds to 8 µm2. Bar, 100 µm.
Figure 3.
BopN suppresses inflammatory responses at the bacterial-colonized area. (A and B) Scanning electron micrographs of mouse tracheas infected with WT (A) and ΔBopN Bordetella (B). C57BL/6J mice were infected intranasally with 5 × 106 WT and ΔBopN B. bronchiseptica, and tracheal sections were obtained 2 d after infection. Arrowheads indicate bacteria. Arrows indicate erythrocytes and inflammatory cells. Note that extensive cell-surface disruption, including increased unciliated cells as well as infiltration of inflammatory cells and erythrocytes, is observed in mice infected with ΔBopN but not WT. The areas of the enlarged boxes correspond to 20 µm2 (top) and 4 µm2 (bottom). Bars: (top and middle) 10 µm; (bottom) 1 µm. (C) DC2.4 cells were infected with the indicated strains (m.o.i. = 10) for 60, 120, and 180 min and then analyzed with Nomarski imaging. Both WT and ΔBopN induced significant morphological changes, including rounding and detachment, in the cells. Bar, 20 µm. (D) Time course of the release of LDH into the extracellular medium from DC2.4 cells infected with the indicated strains. The values are the percentages of LDH released from Triton X-100–lysed DC2.4 cells after subtraction of the value measured in uninfected cells. Error bars represent means ± SE from triplicate experiments. The amounts of LDH released after ΔBopN infection were similar to those released after WT infection.
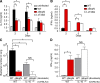
Figure 4.
BopN induces IL-10 production in vivo. (A and B) Detection of IL-10 (A) or IFN-γ (B) production in _Bordetella_-infected mice is shown. C57BL/6J mice (n = 9 for each group) were infected intranasally with 5 × 105 B. bronchiseptica. Homogenized lung specimens were prepared from mice infected with the indicated strains at 2, 5, or 8 d after infection, and the amounts of cytokines in the specimens were determined by ELISA. (C) C57BL/6J (WT) or IL-10−/− mice (n = 3 for each group) on a C57BL/6J background were infected intranasally with the indicated strains (5 × 105 bacteria). 2 d after infection, lung specimens were homogenized and plated on BG agar plates to detect the number of colonizing bacteria. (D) IFN-γ production in the lungs of _Bordetella_-infected mice (n = 3 for each group). Homogenized lung specimens were prepared and the amounts of cytokines were determined by ELISA. The values are means ± SE from three independent experiments. *, P < 0.05.
Figure 5.
CD11c+ cells migrate into the lung and produce IL-10 during Bordetella infection. (A) C57BL/6J mice were infected intranasally with 5 × 105 WT or ΔBopN B. bronchiseptica. 2 d after infection, lung sections were immunostained with anti-CD11c monoclonal antibodies (brown). Note that extensive recruitment of CD11c+ cells into the pulmonary alveoli was observed in mice infected with WT and ΔBopN. Bar, 50 µm. (B) 2 d after infection, the total numbers of CD11c+ cells in the lungs of mice (n = 3 for each group) infected with the indicated strains were determined by FACS analysis. (C) The lung cells from mice (n = 3 for each group) infected with Bordetella were pooled, stained with FITC-conjugated anti–IL-10 and PE-conjugated anti-CD11c, and determined by FACS analysis (percentages are shown). (D) The histogram shows the results obtained in C expressed as a ratio of IL-10+/CD11c+ cells. The values in B and D are means ± SE from three independent experiments. *, P < 0.05.
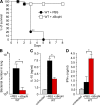
Figure 6.
In vivo successive infection study. (A) C57BL/6J mice (n = 27 for WT + PBS and WT + ΔBopN) were infected intranasally with 5 × 105 ΔBopN or PBS (mock infection) 24 h before infection with a lethal dose of WT Bordetella (9 × 105), and the survival was monitored for 8 d. **, P < 0.005 using the log-rank test. (B) 2 d after the WT lethal-dose infection, the lung specimens of mice (n = 3 for WT + PBS preinfection and WT + ΔBopN preinfection) were homogenized and plated on BG agar plates to detect the number of colonized bacteria. (C and D) 2 d after the WT lethal-dose infection, homogenized lung specimens were prepared from mice (n = 3 for WT + PBS and WT + ΔBopN) infected with the indicated strains, and the amount of IL-10 (C) or IFN-γ (D) in the specimen was determined by ELISA. The values in B–D are means ± SD from three independent experiments. *, P < 0.05.
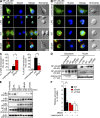
Figure 7.
Molecular characterization of the function of BopN in MAPK pathways. (A) Immunoblot analysis of MAPK activities in the lysates of DC2.4 cells. DC2.4 cells were stimulated with 10 µg/ml LPS or infected with the indicated Bordetella strains for the specified time periods. The lysates were analyzed by Western blotting with antibodies against phospho-p38 (top), phospho-ERK1/2 (third from top), and phospho-JNK (fifth from top). The membranes were stripped and reblotted with antibodies against p38 (second panel), ERK1/2 (fourth from top), and JNK (bottom). (B) Immunofluorescence microscopy of DC2.4 cells stimulated with LPS for 30 min or infected for 30 min with the indicated strains. DC2.4 cells were fixed and stained with anti–phospho-ERK1/2 (green), anti-ERK1/2 (red), and DAPI (blue). Data are representative of three independent experiments. Bar, 20 µm. (C) 10 µM of MEK1/2 inhibitor U0126, 50 µM of p38 kinase inhibitor SB203580, and vehicle control (DMSO) were added to DC2.4 culture medium for 1 h before infection (m.o.i. = 100) with the indicated strains. Total RNA was prepared from the treated cells, and the amounts of IL-10 mRNA produced were assessed by qRT-PCR. (D) DC2.4 cells were infected with the indicated strains (m.o.i. = 100) for the specified time periods, and the amounts of phospho-MEK1/2 and phospho-MKK3/MKK6 were analyzed using immunoblotting. Three independent experiments were performed and a representative immunoblot is shown. (E) DC2.4 cells were stimulated with 10 µg/ml LPS or infected with the indicated strains for 30 min, and nuclear fractions were prepared and analyzed using a Multiplex transcription factor profiling kit. Differences in transcription factor activation were determined by Bio-Plex. The values in C and E are means ± SE from three independent experiments. *, P < 0.05.
Figure 8.
Molecular characterization of the mechanism by which BopN affects NF-κB pathways. (A and B) Immunofluorescence microscopy of DC2.4 cells 30 min after stimulation with 10 µg/ml LPS or infection with WT or ΔBopN Bordetella (m.o.i. = 100). The cells were stained with anti–B. bronchiseptica antibodies (red), anti–NF-κBp65 (A, green), or NF-κBp50 (B, green) antibodies, and DAPI for nuclei (blue). Bars, 10 µm. (C) DC2.4 cells infected with WT or ΔBopN Bordetella were randomly picked from the immunofluorescence micrographs (A and B), and the percentages of cells showing nuclear translocation of NF-κBp65 (left) or NF-κBp50 (right) were determined. Percentages were based on a count of 50 cells, and the values are means ± SD from three independent experiments. *, P < 0.05. (D) DC2.4 cells were stimulated by 10 µg/ml LPS or infected with the indicated strains for 30 min, and then separated into nuclear and cytosolic fractions. The resulting fractions were subjected to immunoblot analyses using anti–NF-κBp65 and anti–NF-κBp50 antibodies. Histone H3 antibodies were used as a nuclear control. The majority of both p65 and p50 was localized in the cytoplasm (A, B, and D) of unstimulated cells. (E) Immunoblot analysis of IκBα, IκBβ, IκB_ϵ_, or NF-κBp105 in the lysates of DC2.4 cells. DC2.4 cells were stimulated with 10 µg/ml LPS or infected with the indicated strains, and total cell lysates were analyzed using immunoblotting with anti-IκBα, anti-IκBβ, anti-IκB_ϵ_, or anti–NF-κBp105 antibodies. (F) 20 nM leptomycin B, an inhibitor of nuclear export, or vehicle control (DMSO) was added to DC2.4 culture medium for 24 h before infection with the indicated strains. Total RNA was prepared from DC2.4 cells, and amounts of IL-10 mRNA were assessed by qRT-PCR. The values are means ± SE from three independent experiments. *, P < 0.05.
Figure 9.
BopN alters the nuclear compartmentalization of NF-κB. (A and B) A mammalian expression vector, pcDNA3.1/His(-)A, encoding the full-length BopN-FL (aa 1–365) or truncated versions of BopN (-NT, aa 1–182; -CT, aa 182–365) with a Myc-tag fused at its C terminus was introduced into Cos7 cells. 24 h after transfection, cells were stimulated with 10 µg/ml LPS for 10 min, and then fixed and stained with anti-Myc antibodies to detect BopN (green), anti–NF-κBp65 (A, red), or NF-κBp50 (B, red), and DAPI for nuclei (blue). Bars, 20 µm. (C) Schematic diagrams of BopN fusion constructs with a Myc-tag (green) used in translocation assays (A and B). aa numbers of full-length and truncated BopN are shown. (D) The pBopN-FL was introduced into RAW264.7 cells. After LPS stimulation for 30 min, BopN-Myc fusion protein and phospho-MAPKs were detected by immunoblot analysis (left). Band intensity of phospho-ERK2 and phospho-p38 were quantified by the ImageJ application (right). The percentages of phospho-MAPK/total MAPK were determined from three independent experiments. (E) The level of IL-10 mRNA in RAW264.7 cells transfected with pBopN-FL was measured by qRT-PCR. The values presented for D and E are means ± SE from three independent experiments. *, P < 0.05.
Similar articles
- BopN is a Gatekeeper of the Bordetella Type III Secretion System.
Navarrete KM, Bumba L, Prudnikova T, Malcova I, Allsop TR, Sebo P, Kamanova J. Navarrete KM, et al. Microbiol Spectr. 2023 Jun 15;11(3):e0411222. doi: 10.1128/spectrum.04112-22. Epub 2023 Apr 10. Microbiol Spectr. 2023. PMID: 37036369 Free PMC article. - Bordetella effector BopN is translocated into host cells via its N-terminal residues.
Abe A, Nishimura R, Kuwae A. Abe A, et al. Microbiol Immunol. 2017 Jun;61(6):206-214. doi: 10.1111/1348-0421.12489. Microbiol Immunol. 2017. PMID: 28500733 - Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system.
Yuk MH, Harvill ET, Cotter PA, Miller JF. Yuk MH, et al. Mol Microbiol. 2000 Mar;35(5):991-1004. doi: 10.1046/j.1365-2958.2000.01785.x. Mol Microbiol. 2000. PMID: 10712682 - Bordetella Type III Secretion Injectosome and Effector Proteins.
Kamanova J. Kamanova J. Front Cell Infect Microbiol. 2020 Sep 4;10:466. doi: 10.3389/fcimb.2020.00466. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33014891 Free PMC article. Review. - Integrated Signaling Pathways Mediate Bordetella Immunomodulation, Persistence, and Transmission.
Gestal MC, Whitesides LT, Harvill ET. Gestal MC, et al. Trends Microbiol. 2019 Feb;27(2):118-130. doi: 10.1016/j.tim.2018.09.010. Epub 2018 Oct 27. Trends Microbiol. 2019. PMID: 30661570 Free PMC article. Review.
Cited by
- Identification of potential nucleomodulins of Mycoplasma bovis by direct biotinylation and proximity-based biotinylation approaches.
Lu D, Chen J, Zhang M, Fu Y, Raheem A, Chen Y, Chen X, Hu C, Chen J, Schieck E, Zhao G, Guo A. Lu D, et al. Front Microbiol. 2024 Jul 9;15:1421585. doi: 10.3389/fmicb.2024.1421585. eCollection 2024. Front Microbiol. 2024. PMID: 39044956 Free PMC article. - Eosinophils as drivers of bacterial immunomodulation and persistence.
Parrish KM, Gestal MC. Parrish KM, et al. Infect Immun. 2024 Sep 10;92(9):e0017524. doi: 10.1128/iai.00175-24. Epub 2024 Jul 15. Infect Immun. 2024. PMID: 39007622 Free PMC article. Review. - T3SS chaperone of the CesT family is required for secretion of the anti-sigma factor BtrA in Bordetella pertussis.
Držmíšek J, Petráčková D, Dienstbier A, Čurnová I, Večerek B. Držmíšek J, et al. Emerg Microbes Infect. 2023 Dec;12(2):2272638. doi: 10.1080/22221751.2023.2272638. Epub 2023 Nov 1. Emerg Microbes Infect. 2023. PMID: 37850324 Free PMC article. - Neutrophil responsiveness to IL-10 impairs clearance of Streptococcus pneumoniae from the lungs.
Horn KJ, Fulte S, Yang M, Lorenz BP, Clark SE. Horn KJ, et al. J Leukoc Biol. 2024 Jan 5;115(1):4-15. doi: 10.1093/jleuko/qiad070. J Leukoc Biol. 2024. PMID: 37381945 Free PMC article. - BopN is a Gatekeeper of the Bordetella Type III Secretion System.
Navarrete KM, Bumba L, Prudnikova T, Malcova I, Allsop TR, Sebo P, Kamanova J. Navarrete KM, et al. Microbiol Spectr. 2023 Jun 15;11(3):e0411222. doi: 10.1128/spectrum.04112-22. Epub 2023 Apr 10. Microbiol Spectr. 2023. PMID: 37036369 Free PMC article.
References
- Arbibe L., Kim D.W., Batsche E., Pedron T., Mateescu B., Muchardt C., Parsot C., Sansonetti P.J. 2007. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 8:47–56 10.1038/ni1423 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases