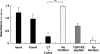Fusobacterium nucleatum envelope protein FomA is immunogenic and binds to the salivary statherin-derived peptide - PubMed (original) (raw)
Fusobacterium nucleatum envelope protein FomA is immunogenic and binds to the salivary statherin-derived peptide
Hidetaka Nakagaki et al. Infect Immun. 2010 Mar.
Abstract
We have previously shown that one of the minimal active regions of statherin, a human salivary protein, for binding to Fusobacterium nucleatum is a YQPVPE amino acid sequence. In this study, we identified the FomA protein of F. nucleatum, which is responsible for binding to the statherin-derived YQPVPE peptide. Overlay analysis showed that a 40-kDa protein of the F. nucleatum cell envelope (40-kDa CE) specifically bound to the YQPVPE peptide. The equilibrium association constant between the affinity-purified 40-kDa CE and the YQPVPE peptide was 4.30 x 10(6). Further, the purity and amino acid sequence analyses of the purified 40-kDa CE revealed approximately 98.7% (wt/wt) purity and a high degree of homology with FomA, a major porin protein of F. nucleatum. Thus, a FomA-deficient mutant failed to bind to the YQPVPE peptide. In addition, increased levels of a FomA-specific mucosal IgA antibody (Ab) and plasma IgG and IgA Abs were seen only in mice immunized nasally with cholera toxin (CT) and the purified 40-kDa FomA protein. Interestingly, saliva from mice that received FomA plus CT as a mucosal adjuvant nasally prevented in vitro binding of F. nucleatum to statherin-coated polyvinyl chloride plates. Taken together, these results suggest that induction of specific immunity to the 40-kDa FomA protein of F. nucleatum, which specifically binds to the statherin-derived peptide, may be an effective tool for preventing the formation of F. nucleatum biofilms in the oral cavity.
Figures
FIG. 1.
The YQPVPE peptide binds to the 40-kDa cell envelope (CE) protein of F. nucleatum. (A) The F. nucleatum (ATCC 25586) CEs that specifically bind to the YQPVPE peptide were determined by a ligand overlay assay. F. nucleatum whole CEs were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was incubated with the biotinylated YQPVPE peptide followed by horseradish peroxidase (HRP)-conjugated streptavidin for substrate development. Lane 1, molecular mass standard marker; lane 2, F. nucleatum whole CEs. (B and C) SDS-PAGE (B) and ligand overlay analysis (C) of F. nucleatum CEs purified by a YQPVPE peptide-coupled, CNBr-activated Sepharose 4B column. Lanes 1 and 3, molecular mass standard marker; lane 2, YQPVPE affinity column-purified F. nucleatum; lane 4, purified F. nucleatum CE incubated with the biotinylated YQPVPE peptide.
FIG. 2.
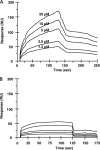
Biomolecular interactions of affinity column-purified F. nucleatum CEs and the YQPVPE peptide. The affinity of binding of the YQPVPE peptide to immobilized F. nucleatum CEs (A) or BSA (B) was estimated by the BIAcore system. HBSP buffer (10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, and 0.005% Tween 20 [pH 7.4]) was used as a running buffer, with a flow rate of 30 μl/min. The statherin peptide solution at various concentrations (1.3 μM to 20 μM) was monitored and injected over the 40-kDa CE (100 μg/ml) or BSA (100 μg/ml) on the CM5 sensor chip.
FIG. 3.
Homology search for the amino acid alignment of the 40-kDa F. nucleatum component. The 40-kDa CE of F. nucleatum was digested with V8 protease, and the amino-terminal sequences of the three cleaved fragments were determined using the BLASTp database program. The underlined sequences correspond to the identified amino-terminal sequences of F. nucleatum FomA and the 40-kDa CE of the F. nucleatum envelope obtained from the YQPVPE peptide-conjugated affinity column.
FIG. 4.
Construction and functional analysis of the Δ_fomA_ mutant strain SN-3. (A) pFOMA151 contains an internal fragment of fomA and a kanamycin resistance gene (aphA3). SN-3 was produced by single-crossover recombination. (B) The Δ_fomA_ mutant strain SN-3 and the wild-type strain of F. nucleatum (ATCC 25586) were subjected to PCR with forward primer fomABamF1 and reverse primer aphA3F2. (C) Dot blot assay for direct binding activity of ATCC 25586 or mutant strain SN-3 to the YQPVPE peptide. The dot blots show typical results for both the experimental (binding to the YQPVPE peptide) and control (binding to BSA) groups. The level of dot blot density for the control group was subtracted from the level of dot blot density for the experimental group in each experiment. The relative percentage of dot density for each was calculated relative to the dot density for wild-type F. nucleatum bound to the YQPVPE peptide, which was defined as 100%. The graph shows the average percentages of dot density for the binding of wild-type F. nucleatum (100%) or SN-3 (21%) to the YQPVPE peptide. The experiments were performed in triplicate on three separate occasions. Data are expressed as means ± standard deviations. The asterisk indicates a significant difference (P < 0.05) from the result for the wild-type strain.
FIG. 5.
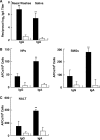
FomA-specific immune responses in external secretions and mucosal lymphoid tissues. C57BL/6 mice were nasally immunized weekly for three consecutive weeks either with FomA protein (20 μg) plus cholera toxin (CT; 1 μg) as a mucosal adjuvant (filled bars) or with FomA only (open bars). (A) Seven days after the last immunization, the levels of FomA-specific IgA Abs in nasal washes and saliva were determined by FomA-specific ELISAs. Data are means ± SEMs (n = 15). Double asterisks indicate significant differences (P < 0.01) from results for control mice. (B and C) Seven days after the last immunization, mononuclear cells isolated from NPs, SMGs, and NALT were subjected to Ag-specific ELISPOT assays in order to determine the numbers of IgG and IgA AFCs. Mice immunized nasally with FomA alone were used as controls. Data are means ± SEMs (n = 15). Asterisks indicate significant differences (**, P < 0.01) from results for control mice.
FIG. 6.
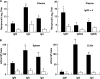
Comparison of FomA-specific Ab responses in systemic lymphoid tissues of mice given FomA with (filled bars) or without (open bars) CT. Each mouse group was immunized nasally weekly for three consecutive weeks. (A) Seven days after the last immunization, FomA-specific IgM, IgG, IgA, and IgG subclass Ab responses in plasma were determined by Ag-specific ELISAs. (B) Seven days after the last immunization, mononuclear cells were isolated from spleens and CLNs and were then subjected to Ag-specific ELISPOT assays in order to determine the numbers of IgM, IgG, and IgA AFCs. Data are means ± SEMs (n = 15). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) from results for control mice.
FIG. 7.
Effect of saliva on the formation of F. nucleatum biofilms on statherin-coated PVC plates. F. nucleatum (5 × 107 bacteria/well) was preincubated with either KCl buffer alone (no inhibitor), saliva from naïve mice, saliva from mice immunized nasally either with FomA alone or with FomA plus CT, or YQPVPE peptide solution at 25°C for 3 h. Subsequently, each mixture, as well as a sample with no bacteria, was added to statherin-coated PVC plates and incubated at 25°C overnight. The resulting biofilm was stained with crystal violet and extracted with 95% ethanol. Biofilm formation was scored by measuring the absorbance at 595 nm. All assays were performed in triplicate, and means and standard deviations are shown. Asterisks indicate significant differences (**, P < 0.01) from results for control mice.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Lung mucosal immunity to NTHi vaccine antigens: Antibodies in sputum of chronic obstructive pulmonary disease patients.
Baffetta F, Buonsanti C, Moraschini L, Aprea S, Canè M, Lombardi S, Contorni M, Rondini S, Arora AK, Bardelli M, Finco O, Serruto D, Paccani SR. Baffetta F, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2343544. doi: 10.1080/21645515.2024.2343544. Epub 2024 Apr 24. Hum Vaccin Immunother. 2024. PMID: 38655676 Free PMC article. Clinical Trial. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article. - Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.
Harnan S, Kearns B, Scope A, Schmitt L, Jankovic D, Hamilton J, Srivastava T, Hill H, Ku CC, Ren S, Rothery C, Bojke L, Sculpher M, Woods B. Harnan S, et al. Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
- The FomA porin from Fusobacterium nucleatum is a Toll-like receptor 2 agonist with immune adjuvant activity.
Toussi DN, Liu X, Massari P. Toussi DN, et al. Clin Vaccine Immunol. 2012 Jul;19(7):1093-101. doi: 10.1128/CVI.00236-12. Epub 2012 May 23. Clin Vaccine Immunol. 2012. PMID: 22623652 Free PMC article. - Role of FAD-I in Fusobacterial Interspecies Interaction and Biofilm Formation.
Shokeen B, Park J, Duong E, Rambhia S, Paul M, Weinberg A, Shi W, Lux R. Shokeen B, et al. Microorganisms. 2020 Jan 2;8(1):70. doi: 10.3390/microorganisms8010070. Microorganisms. 2020. PMID: 31906541 Free PMC article. - Mucosal Vaccination Against Periodontal Disease: Current Status and Opportunities.
Vaernewyck V, Arzi B, Sanders NN, Cox E, Devriendt B. Vaernewyck V, et al. Front Immunol. 2021 Dec 2;12:768397. doi: 10.3389/fimmu.2021.768397. eCollection 2021. Front Immunol. 2021. PMID: 34925337 Free PMC article. Review. - Outer Membrane Vesicles From Fusobacterium nucleatum Switch M0-Like Macrophages Toward the M1 Phenotype to Destroy Periodontal Tissues in Mice.
Chen G, Sun Q, Cai Q, Zhou H. Chen G, et al. Front Microbiol. 2022 Mar 21;13:815638. doi: 10.3389/fmicb.2022.815638. eCollection 2022. Front Microbiol. 2022. PMID: 35391731 Free PMC article. - Fusobacterium nucleatum - symbiont, opportunist and oncobacterium.
Brennan CA, Garrett WS. Brennan CA, et al. Nat Rev Microbiol. 2019 Mar;17(3):156-166. doi: 10.1038/s41579-018-0129-6. Nat Rev Microbiol. 2019. PMID: 30546113 Free PMC article. Review.
References
- Couch, R. B. 2004., Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N. Engl. J. Med. 350:860-861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE 012242/DE/NIDCR NIH HHS/United States
- AG 025873/AG/NIA NIH HHS/United States
- R01 DE012242/DE/NIDCR NIH HHS/United States
- R29 DE012242/DE/NIDCR NIH HHS/United States
- R01 AG025873/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous