Rab13 regulates neurite outgrowth in PC12 cells through its effector protein, JRAB/MICAL-L2 - PubMed (original) (raw)
Rab13 regulates neurite outgrowth in PC12 cells through its effector protein, JRAB/MICAL-L2
Ayuko Sakane et al. Mol Cell Biol. 2010 Feb.
Abstract
Neurite outgrowth is the first step in the processes of neuronal differentiation and regeneration and leads to synaptic polarization and plasticity. Rab13 small G protein shows an increased mRNA expression level during neuronal regeneration; it is therefore thought to be involved in this process. We previously identified JRAB (junctional Rab13-binding protein)/MICAL-L2 (molecules interacting with CasL-like 2) as a novel Rab13 effector protein. Here, we show that Rab13 regulates neurite outgrowth in the rat pheochromocytoma cell line PC12 through an interaction with JRAB/MICAL-L2. The expression of JRAB/MICAL-L2 alone inhibits neurite outgrowth, whereas coexpression of the dominant active form of Rab13 rescues this effect. We also demonstrate an intramolecular interaction between the N-terminal calponin-homology (CH) and LIM domains of JRAB/MICAL-L2 and the C-terminal coiled-coil domain. Finally, we show that the binding of Rab13 to JRAB/MICAL-L2 stimulates the interaction of JRAB/MICAL-L2 with actinin-4, an actin-binding protein, which localizes to the cell body and the tips of the neurites in PC12 cells. These results suggest that Rab13 and JRAB/MICAL-L2 may act to transfer actinin-4 from the cell body to the tips of neurites, where actinin-4 is involved in the reorganization of the actin cytoskeleton which results in neurite outgrowth.
Figures
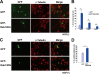
FIG. 1.
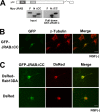
The dominant active mutant of Rab13 induces neurite outgrowth in PC12 cells. (A) PC12 cells were transfected with an expression vector encoding GFP alone (GFP, upper panels) or GFP-tagged dominant active mutant of Rab13 (GFP-Rab13DA, lower panels). After 48 h, cells were fixed and immunostained with anti-β-tubulin antibody. Bar, 20 μm. (B) More than 100 transfected cells per experiment (random) were blindly selected, and the lengths of their neurites were measured by using ImageJ software (National Institutes of Health). The mean values (± the standard error) of the percentages of cells bearing neurites longer than 20, 40, or 60 μm from three independent experiments are shown. Statistical analyses were performed by analysis of variance (ANOVA) accompanied by a post-hoc Scheffe test. *, P < 0.05. (C) PC12 cells were transfected with an expression vector encoding GFP alone (GFP, upper panels) or GFP-tagged dominant negative mutant of Rab13 (GFP-Rab13DN, lower panels). Cells were visualized by immunostaining with an antibody against β-tubulin as described in panel A. Bar, 20 μm. (D) More than 100 transfected cells per experiment (random) were blindly selected, and the number of undifferentiated cells was determined. The histogram shows the percentage of undifferentiated cells in each group from three independent experiments. Statistical analyses were performed by using Student's t test. *, P < 0.05.
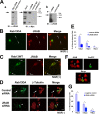
FIG. 2.
JRAB/MICAL-L2 is required for Rab13-mediated neurite outgrowth in PC12 cells. (A) In subpanel a, left panel, COS7 cells were transfected with Myc-tagged JRAB/MICAL-L2 (lane 1) or empty vector (lane 2), lysed, and immunoblotted with an anti-JRAB/MICAL-L2 antibody. For the right panel, immunoprecipitation from the lysates of COS7 cells expressing Myc-tagged JRAB/MICAL-L2 (lane 1, input) was performed with an anti-JRAB/MICAL-L2 antibody (lane 2), control rabbit IgG (lane 3), or no antibody (lane 4). Immunoprecipitates were examined by immunoblotting with an anti-Myc antibody. In this blot, 5.5% of the total protein was loaded as an input. Subpanel b shows the expression of JRAB/MICAL-L2 in PC12 cells. Endogenous JRAB/MICAL-L2 was immunoprecipitated from PC12 cell lysates with an anti-JRAB/MICAL-L2 antibody (lane 1) but not with a control antibody (lane 2). An arrow indicates JRAB/MICAL-L2. (B) PC12 cells expressing GFP-Rab13DA were fixed, stained for JRAB/MICAL-L2 (red) with an anti-JRAB/MICAL-L2 antibody, and observed using fluorescence microscopy. GFP-Rab13DA and JRAB/MICAL-L2 were colocalized to the cell body and the tips of the neurites (arrows). Bar, 20 μm. (C) PC12 cells transfected with an expression vector encoding GFP-tagged wild-type Rab13 (GFP-Rab13WT) and treated with NGF were fixed and processed for immunostaining with an anti-JRAB/MICAL-L2 antibody (red). Endogenous JRAB/MICAL-L2 was mainly colocalized with GFP-Rab13WT in the perinuclear region and at the tips of the neurites (arrows). Bar, 10 μm. (D) PC12 cells were transfected with siRNA#3 or control siRNA combined with pEGFP-Rab13DA. siRNA#3 inhibited Rab13DA-mediated neurite outgrowth compared to control siRNA. Bar, 20 μm. (E) Quantification of neurite lengths in cells cotransfected with pEGFP-Rab13DA and siRNA#3 or control siRNA. Columns represent the percentage of cells bearing neurites longer than 20, 40, or 60 μm from three independent experiments (n ≥ 100 per experiment). Statistical analyses were performed by ANOVA accompanied by a post-hoc Scheffe test. *, P < 0.05. (F) PC12 cells transfected with pcDNA6.2-GW/EmGFP-miR vector containing JRAB/MICAL-L2-directed miRNA insert, which was designed based on the target sequence of siRNA #3, were fixed and processed for immunostaining with an anti-JRAB/MICAL-L2 antibody (red). The staining of endogenous JRAB/MICAL-L2 was reduced in the transfected cells (arrowhead). Bar, 10 μm. (G) PC12 cells were transfected with pcDNA6.2-GW/EmGFP-miR-JRAB/MICAL-L2 #3 or pcDNA6.2-GW/EmGFP-miR-neg control plasmid, followed by treatment with NGF. The histogram shows the percentage of cells bearing neurites longer than 10, 20, or 40 μm from three independent experiments (n ≥ 100 per experiment). Statistical analyses were performed by ANOVA accompanied by a post-hoc Scheffe test. *, P < 0.05.
FIG. 3.
Neurite outgrowth is inhibited by overexpressed JRAB/MICAL-L2 in PC12 cells. (A) PC12 cells transfected with pEGFP-JRAB/MICAL-L2 or pEGFP and treated with NGF were fixed and processed for immunostaining with an anti-β-tubulin antibody. GFP-JRAB/MICAL-L2 accumulated at the perinuclear region (arrowheads) and inhibited neurite outgrowth in all of the transfected cells (n = 266). Bar, 20 μm. (B) PC12 cells cotransfected with pEGFP-JRAB/MICAL-L2 and pDsRed-Rab13DA or pDsRed were fixed and processed for immunostaining with an anti-β-tubulin antibody. Cotransfection with pEGFP-JRAB/MICAL-L2 and pDsRed-Rab13DA led to neurite outgrowth (arrows) and disrupted the accumulation of GFP-JRAB/MICAL-L2 at the perinuclear region (n = 248). Bar, 20 μm.
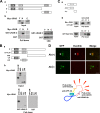
FIG. 4.
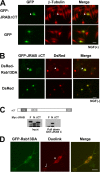
Intramolecular interaction between the N-terminal and C-terminal regions of JRAB/MICAL-L2. (A) The N-terminal region of JRAB/MICAL-L2 interacts with its C-terminal region. (a) Structures of the full-length JRAB/MICAL-L2 (row F) and JRAB/MICAL-L2 mutants: JRAB-N (row N) and JRAB-C (row C). JRAB/MICAL-L2 is composed of the CH domain (CH, amino acids 3 to 102), the LIM domain (LIM, amino acids 131 to 260), and the CC domain (CC, amino acids 806 to 912). (b) Pulldown assay. COS7 cells expressing Myc-tagged JRAB/MICAL-L2 constructs (F, N, and C) were lysed and subjected to pulldown assay with GST-JRAB-C. Bound proteins were detected by immunoblotting with an anti-Myc antibody. CBB indicates the Coomassie brilliant blue staining of GST-JRAB-C and GST used for the assays. Myc-JRAB-N bound to GST-JRAB-C, but Myc-JRAB-F and -JRAB-C did not. (B) The CH+LIM domain of JRAB/MICAL-L2 interacts with its C-terminal region. (a) Structures of various fragments of JRAB-N. (b) COS7 cells expressing the indicated truncated mutants (CH+LIM, MID, CH, and LIM) were lysed and subjected to the pulldown assay using GST-JRAB-C. Bound proteins were detected by immunoblotting with an anti-Myc antibody. Myc-JRAB-CH+LIM and -LIM bound to GST-JRAB-C, but Myc-JRAB-CH and -MID did not. (C) The Rab13- and JRAB-CH+LIM-interacting domains were separable. (a) Structures of various fragments of JRAB-C. (b) COS7 cells expressing Myc-JRAB-CH+LIM or GFP-Rab13DA were lysed and subjected to the pulldown assay using GST-JRAB-C, -CC, or -CT. The bound proteins were detected by immunoblotting with anti-Myc and anti-GFP antibodies. Myc-JRAB-CH+LIM bound to GST-JRAB-C and -CC, whereas GFP-Rab13DA bound to GST-JRAB-C and -CT. (D) Intramolecular interaction of JRAB/MICAL-L2 was observed by Duolink assay in PC12 cells. PC12 cells transfected with GFP-JRAB/MICAL-L2 were fixed and subjected to Duolink in situ PLA. The assays were performed with an anti-GFP antibody in the presence [Ab(+)] or absence [Ab(−)] of an anti-JRAB/MICAL-L2 antibody. An arrowhead in the upper panel indicates a positive signal. Bar, 20 μm. The schematic illustrates the principle of the Duolink in situ PLA; the anti-GFP and anti-JRAB/MICAL-L2 antibodies bound to GFP-JRAB/MICAL-L2 expressed in PC12 cells in close proximity, producing the positive signal.
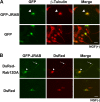
FIG. 5.
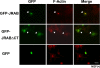
The JRABΔCT mutant inhibits Rab13-mediated neurite outgrowth in PC12 cells. (A) PC12 cells were transfected with either pEGFP-JRABΔCT or pEGFP and stimulated with NGF for 2 days, followed by immunostaining for β-tubulin. Accumulation of GFP-JRABΔCT (arrowheads) was observed in all of the transfected cells (n = 279). Bar, 20 μm. (B) PC12 cells cotransfected with pEGFP-JRABΔCT and pDsRed-Rab13DA or pDsRed were observed by direct GFP or DsRed fluorescence (n ≥ 100 per experiment). Bar, 20 μm. (C) Schematic of the structure of JRABΔCT, lacking the CT domain (amino acids 913 to 1009). COS7 cells expressing Myc-JRAB-F, -N, or -ΔCT were lysed and subjected to the pulldown assay using GST-JRAB-C. Neither Myc-JRABΔCT nor Myc-JRAB-F bound to GST-JRAB-C, whereas Myc-JRAB-N did. (D) PC12 cells transfected with GFP-Rab13DA were fixed and subjected to the Duolink in situ PLA. The assay was performed with anti-GFP and anti-JRAB/MICAL-L2 antibodies. A positive signal was observed in both the cell body (arrowhead) and neurites (arrow). Bar, 20 μm.
FIG. 6.
Effect of JRABΔCC mutant on neurite outgrowth in PC12 cells. (A) Schematic of the structure of JRABΔCC, lacking the CC domain (amino acids 806 to 912). COS7 cells expressing Myc-JRAB-F, -N, or -ΔCC were lysed and subjected to the pulldown assay using GST-JRAB-C. Either Myc-JRABΔCC or Myc-JRAB-N bound to GST-JRAB-C, whereas Myc-JRAB-F did not. (B) PC12 cells were transfected with pEGFP-JRABΔCC and immunostained with anti-β-tubulin antibody. Accumulation of GFP-JRABΔCC was not observed in all of the transfected cells (n = 198). Bar, 20 μm. (C) PC12 cells cotransfected with pEGFP-JRABΔCC and pDsRed-Rab13DA or pDsRed were observed by direct GFP or DsRed fluorescence (n ≥ 100 per experiment). Bar, 20 μm.
FIG. 7.
Effect of JRAB/MICAL-L2 on actin cytoskeletal rearrangement during neurite outgrowth in PC12 cells. PC12 cells transfected with pEGFP-JRAB/MICAL-L2, pEGFP-JRABΔCT, or pEGFP were fixed and processed for double fluorescence to visualize the actin filaments by direct GFP fluorescence detection and indirect immunofluorescence detection using rhodamine-phalloidin. GFP-JRAB/MICAL-L2 and GFP-JRABΔCT (arrows) were accumulated in the perinuclear region and costained with phalloidin in all of the transfected cells (n ≥ 100 per experiment). Bar, 20 μm.
FIG. 8.
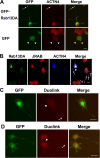
Rab13-JRAB/MICAL-L2-actinin-4 is involved in neurite outgrowth in PC12 cells. (A) PC12 cells transfected with pEGFP-Rab13DA or pEGFP were fixed and stained with an anti-actinin-4 antibody. Actinin-4 was colocalized with GFP-Rab13DA in both the cell body and the tips of the Rab13-induced neurites (arrows). Bar, 20 μm. (B) PC12 cells cotransfected with pEGFP-Rab13DA and pCIneo-Myc-actinin-4 were fixed and stained with anti-JRAB/MICAL-L2 and anti-Myc antibodies. Actinin-4 was colocalized with GFP-Rab13DA and JRAB/MICAL-L2 in both the cell body (arrowhead) and the tips of the Rab13-induced neurites (arrows). Bar, 20 μm. (C) PC12 cells cotransfected with pEGFP-Rab13DA and pCIneo-Myc-actinin-4 were fixed and subjected to the Duolink assay. The assay was performed with anti-Myc and anti-JRAB/MICAL-L2 antibodies. A positive signal was observed in both the cell body (arrowhead) and neurites (arrow). Bar, 20 μm. (D) PC12 cells cotransfected with pCIneo-HA-Rab13DA, pEGFP-JRAB/MICAL-L2 and pCIneo-Myc-actinin-4 were fixed and subjected to the Duolink assay. The assay was performed with anti-Myc and anti-GFP antibodies. The signal was stronger than that observed in panel C. Bar, 20 μm.
Similar articles
- Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions.
Nakatsuji H, Nishimura N, Yamamura R, Kanayama HO, Sasaki T. Nakatsuji H, et al. Mol Cell Biol. 2008 May;28(10):3324-35. doi: 10.1128/MCB.00144-08. Epub 2008 Mar 10. Mol Cell Biol. 2008. PMID: 18332111 Free PMC article. - Actin Cytoskeletal Reorganization Function of JRAB/MICAL-L2 Is Fine-tuned by Intramolecular Interaction between First LIM Zinc Finger and C-terminal Coiled-coil Domains.
Miyake K, Sakane A, Tsuchiya Y, Sagawa I, Tomida Y, Kasahara J, Imoto I, Watanabe S, Higo D, Mizuguchi K, Sasaki T. Miyake K, et al. Sci Rep. 2019 Sep 5;9(1):12794. doi: 10.1038/s41598-019-49232-8. Sci Rep. 2019. PMID: 31488862 Free PMC article. - The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions.
Yamamura R, Nishimura N, Nakatsuji H, Arase S, Sasaki T. Yamamura R, et al. Mol Biol Cell. 2008 Mar;19(3):971-83. doi: 10.1091/mbc.e07-06-0551. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094055 Free PMC article. - Regulation of epithelial cell adhesion and repulsion: role of endocytic recycling.
Nishimura N, Sasaki T. Nishimura N, et al. J Med Invest. 2008 Feb;55(1-2):9-16. doi: 10.2152/jmi.55.9. J Med Invest. 2008. PMID: 18319540 Review. - Structure-function studies of MICAL, the unusual multidomain flavoenzyme involved in actin cytoskeleton dynamics.
Vanoni MA. Vanoni MA. Arch Biochem Biophys. 2017 Oct 15;632:118-141. doi: 10.1016/j.abb.2017.06.004. Epub 2017 Jun 8. Arch Biochem Biophys. 2017. PMID: 28602956 Review.
Cited by
- JRAB/MICAL-L2 undergoes liquid-liquid phase separation to form tubular recycling endosomes.
Sakane A, Yano TA, Uchihashi T, Horikawa K, Hara Y, Imoto I, Kurisu S, Yamada H, Takei K, Sasaki T. Sakane A, et al. Commun Biol. 2021 May 11;4(1):551. doi: 10.1038/s42003-021-02080-7. Commun Biol. 2021. PMID: 33976349 Free PMC article. - Novel interaction of Rab13 and Rab8 with endospanins.
Hirvonen MJ, Büki KG, Sun Y, Mulari MT, Härkönen PL, Väänänen KH. Hirvonen MJ, et al. FEBS Open Bio. 2013 Jan 22;3:83-8. doi: 10.1016/j.fob.2013.01.004. Print 2013. FEBS Open Bio. 2013. PMID: 23772379 Free PMC article. - Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation.
Pope SM, Lässer C. Pope SM, et al. J Extracell Vesicles. 2013 Dec 11;2. doi: 10.3402/jev.v2i0.22484. eCollection 2013. J Extracell Vesicles. 2013. PMID: 24363837 Free PMC article. - MICAL-like1 mediates epidermal growth factor receptor endocytosis.
Abou-Zeid N, Pandjaitan R, Sengmanivong L, David V, Le Pavec G, Salamero J, Zahraoui A. Abou-Zeid N, et al. Mol Biol Cell. 2011 Sep;22(18):3431-41. doi: 10.1091/mbc.E11-01-0030. Epub 2011 Jul 27. Mol Biol Cell. 2011. PMID: 21795389 Free PMC article. - Regulation of Cancer Cell Behavior by the Small GTPase Rab13.
Ioannou MS, McPherson PS. Ioannou MS, et al. J Biol Chem. 2016 May 6;291(19):9929-37. doi: 10.1074/jbc.R116.715193. Epub 2016 Apr 4. J Biol Chem. 2016. PMID: 27044746 Free PMC article. Review.
References
- Buck, M., W. Xu, and M. K. Rosen. 2004. A two-state allosteric model for autoinhibition rationalizes WASP signal integration and targeting. J. Mol. Biol. 338:271-285. - PubMed
- Dent, E. W., and F. B. Gertler. 2003. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40:209-227. - PubMed
- Dickson, B. J. 2001. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 11:103-110. - PubMed
- Di Giovanni, S., A. De Biase, A. Yakovlev, T. Finn, J. Beers, E. P. Hoffman, and A. I. Faden. 2005. In vivo and in vitro characterization of novel neuronal plasticity factors identified following spinal cord injury. J. Biol. Chem. 280:2084-2091. - PubMed
- Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases