Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas - PubMed (original) (raw)
Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas
Ping Mu et al. Genes Dev. 2009.
Abstract
The miR-17 approximately 92 cluster is frequently amplified or overexpressed in human cancers and has emerged as the prototypical oncogenic polycistron microRNA (miRNA). miR-17 approximately 92 is a direct transcriptional target of c-Myc, and experiments in a mouse model of B-cell lymphomas have shown cooperation between these two oncogenes. However, both the molecular mechanism underlying this cooperation and the individual miRNAs that are responsible for it are unknown. By using a conditional knockout allele of miR-17 approximately 92, we show here that sustained expression of endogenous miR-17 approximately 92 is required to suppress apoptosis in Myc-driven B-cell lymphomas. Furthermore, we show that among the six miRNAs that are encoded by miR-17 approximately 92, miR-19a and miR-19b are absolutely required and largely sufficient to recapitulate the oncogenic properties of the entire cluster. Finally, by combining computational target prediction, gene expression profiling, and an in vitro screening strategy, we identify a subset of miR-19 targets that mediate its prosurvival activity.
Figures
Figure 1.
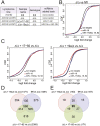
miR-17∼92 suppresses cell death in Eμ-Myc lymphomas. (A) Schematic representation of the miR-17∼92 cluster. Each miRNA is represented by a colored box and is color-coded based on the seed family to which it belongs. The sequence of each mature miRNA is also shown. (B) Schematic of the conditional miR-17∼92 knockout allele. Arrows represent the primers used to detect the floxed and the deleted (Δ) alleles. (C) PCR on genomic DNA extracted from Eμ-Myc;miR-17∼92fl/fl;Cre-ER lymphoma cells mock-treated or after 4 d of 4-OHT treatment. (D) Quantitative RT–PCR analysis of the expression of miR-17∼92 in lymphoma cells before (gray bars) and after (white bars) 4-OHT treatment. Each component of miR-17∼92 was detected independently, and the results were normalized relative to the expression observed in mock-treated cells. Each experiment was performed in quadruplicate. Error bar, standard deviation (SD). (E) Growth curves of _miR-17∼92_fl/fl cells (black line), miR-17∼92Δ/Δ cells (red line), and miR-17∼92Δ/Δ cells infected with a retrovirus expressing the entire miR-17∼92 cluster (blue line). Error bars, SD of three replicates. The plot is representative of three independent experiments. (F) Caspase activity in exponentially growing _miR-17∼92_fl/fl and miR-17∼92Δ/Δ lymphoma cells as detected by flow cytometry using FITC-conjugated VAD-FMK. The percent of VAD-FMK+ cells is shown.
Figure 2.
miR-19a and miR-19b mediate the prosurvival and oncogenic functions of miR-17∼92 in Eμ-Myc B-cell lymphomas. (A) Schematic of the experimental design. (B) Histogram overlays of miR-17∼92Δ/Δ cells transduced with PIG retroviruses expressing the indicated miR-17∼92 derivatives. The cells were assayed by flow cytometry to detect GFP expression at day 2 (blue plot) and day 8 (red plot) post-infection. A schematic of the miR-17∼92 derivative used is shown below each overlay. (C) Table summarizing the results of the experiments shown in B. (D) Caspase activity in _miR-17∼92_fl/fl and miR-17∼92Δ/Δ cells transduced with the indicated PIG constructs. Error bar, 1 SD deviation. (E) Survival analysis of mice injected with _miR-17∼92_fl/fl and miR-17∼92Δ/Δ lymphoma cells transduced with the indicated PIG constructs. N = 16 mice for each construct, over three independent experiments.
Figure 3.
Gene expression profiling identifies miR-19 targets in Eμ-Myc lymphoma cells. (A) Description of the various lymphoma cells used. (B) Differences in mRNA levels between _miR-17∼92_fl/fl and miR-17∼92Δ/Δ lymphoma cells transduced with the empty PIG vector were monitored with microarrays. Cumulative distribution function (CDF) plots are shown for mRNAs that do not contain miR-17∼92 seed matches in their 3′UTRs (black line), mRNAs containing one or more seed matches for miR-19 in their 3′UTR (red line), and mRNAs containing one or more seed matches for either miR-17, miR-20a, miR-18a, or miR-92 (blue line). In the absence of endogenous miR-17∼92 expression, a statistically significant up-regulation (_P_-value < 2.22e-16, KS test) is observed for the predicted miR-17∼92 targets relative to the background gene population. (C) CDF plots of the changes in mRNA expression levels between miR-17∼92Δ/Δ + PIG-miR-17∼92 and miR-17∼92Δ/Δ lymphoma cells (left panel) and between miR-17∼92Δ/Δ + PIG-miR-19a,b and miR-17∼92Δ/Δ lymphoma cells (right panel). (D) Venn diagram summarizing the overlap in gene expression changes observed between the various transduction experiments. (E) As in D, but the analysis was restricted to mRNAs whose 3′UTR contains at least one predicted binding site for miR-19.
Figure 4.
An in vitro RNAi screen to identify functionally relevant miR-19 targets. (A) List of the genes assayed in the in vitro shRNA screen. (B) Scatter plot summarizing the result of the screen. Each dot represents an individual shRNA construct. The _X_-axis is the percentage of GFP cells at the beginning of the experiment (2 d after infection) and the _Y_-axis is the percentage of GFP cells 11 d later. The green dot identifies the empty vector control. shRNAs that scored positive in the screen are highlighted in red and labeled. Dots corresponding to genes for which at least two out of three shRNAs provided significant growth advantage are labeled. PIG-miR-19a/b was included in the screen as positive control (blue dot).
Figure 5.
Pten is a functionally relevant miR-19 target in B-cell lymphomas. (A) Schematic representation of the Pten 3′UTR with the location of the predicted binding sites for members of the miR-17∼92 cluster and sequence alignments between miR-19b and its two predicted binding sites. (B) Pten Western blot on lysates of B-lymphoma cells transduced with the indicated PIG constructs. (Lanes 8,9) For comparison, lysates from miR-17∼92Δ/Δ cell expressing the two Pten shRNAs that scored positive in the in vitro screen were also assayed. (C) Pten immunohistochemistry on lymphoma sections obtained from mice injected with _miR-17∼92_fl/fl and miR-17∼92Δ/Δ B-lymphoma cells transduced with the indicated miR-17∼92 derivatives (objective, 20×). Brown staining indicates Pten signal. (D) Knockdown of Pten suppresses apoptosis in miR-17∼92Δ/Δ cells. Apoptosis was measured by detecting caspase activity in miR-17∼92fl/fl and miR-17∼92Δ/Δ cells transduced with the indicated retroviruses. (E) Kaplan-Meier survival curve of mice injected with miR-17∼92Δ/Δ lymphoma cells transduced with retroviruses expressing shRNAs against Pten. N = 10 (five mice for shPTEN-1 and five mice for shPTEN-2). For comparison, the survival curves of mice injected with _miR-17∼92_fl/fl, miR-17∼92Δ/Δ, and miR-17∼92Δ/Δ + miR-19a,b from Figure 2C are included.
Comment in
- Tumorigenicity of the miR-17-92 cluster distilled.
van Haaften G, Agami R. van Haaften G, et al. Genes Dev. 2010 Jan 1;24(1):1-4. doi: 10.1101/gad.1887110. Genes Dev. 2010. PMID: 20047995 Free PMC article.
Similar articles
- A microRNA polycistron as a potential human oncogene.
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. He L, et al. Nature. 2005 Jun 9;435(7043):828-33. doi: 10.1038/nature03552. Nature. 2005. PMID: 15944707 Free PMC article. - Synergistic action of the microRNA-17 polycistron and Myc in aggressive cancer development.
Tagawa H, Karube K, Tsuzuki S, Ohshima K, Seto M. Tagawa H, et al. Cancer Sci. 2007 Sep;98(9):1482-90. doi: 10.1111/j.1349-7006.2007.00531.x. Epub 2007 Jun 30. Cancer Sci. 2007. PMID: 17608773 Free PMC article. - Targeting miR-21 with NL101 blocks c-Myc/Mxd1 loop and inhibits the growth of B cell lymphoma.
Li S, He X, Gan Y, Zhang J, Gao F, Lin L, Qiu X, Yu T, Zhang X, Chen P, Tong J, Qian W, Xu Y. Li S, et al. Theranostics. 2021 Jan 19;11(7):3439-3451. doi: 10.7150/thno.53561. eCollection 2021. Theranostics. 2021. PMID: 33537096 Free PMC article. - MicroRNAs in B-cell lymphomas: how a complex biology gets more complex.
Musilova K, Mraz M. Musilova K, et al. Leukemia. 2015 May;29(5):1004-17. doi: 10.1038/leu.2014.351. Epub 2014 Dec 26. Leukemia. 2015. PMID: 25541152 Review. - c-MYC-miRNA circuitry: a central regulator of aggressive B-cell malignancies.
Tao J, Zhao X, Tao J. Tao J, et al. Cell Cycle. 2014;13(2):191-8. doi: 10.4161/cc.27646. Epub 2014 Jan 6. Cell Cycle. 2014. PMID: 24394940 Free PMC article. Review.
Cited by
- Neurobehavioral Alterations in a Genetic Murine Model of Feingold Syndrome 2.
Fiori E, Babicola L, Andolina D, Coassin A, Pascucci T, Patella L, Han YC, Ventura A, Ventura R. Fiori E, et al. Behav Genet. 2015 Sep;45(5):547-59. doi: 10.1007/s10519-015-9724-8. Epub 2015 May 31. Behav Genet. 2015. PMID: 26026879 Free PMC article. - Analysis of transcriptional regulation of the human miR-17-92 cluster; evidence for involvement of Pim-1.
Thomas M, Lange-Grünweller K, Hartmann D, Golde L, Schlereth J, Streng D, Aigner A, Grünweller A, Hartmann RK. Thomas M, et al. Int J Mol Sci. 2013 Jun 7;14(6):12273-96. doi: 10.3390/ijms140612273. Int J Mol Sci. 2013. PMID: 23749113 Free PMC article. - MicroRNA-17-5p post-transcriptionally regulates p21 expression in irradiated betel quid chewing-related oral squamous cell carcinoma cells.
Wu SY, Lin KC, Chiou JF, Jeng SC, Cheng WH, Chang CI, Lin WC, Wu LL, Lee HL, Chen RJ. Wu SY, et al. Strahlenther Onkol. 2013 Aug;189(8):675-83. doi: 10.1007/s00066-013-0347-9. Epub 2013 Jun 20. Strahlenther Onkol. 2013. PMID: 23780339 - Mouse hospital and co-clinical trial project--from bench to bedside.
Clohessy JG, Pandolfi PP. Clohessy JG, et al. Nat Rev Clin Oncol. 2015 Aug;12(8):491-8. doi: 10.1038/nrclinonc.2015.62. Epub 2015 Apr 21. Nat Rev Clin Oncol. 2015. PMID: 25895610 Review. - Myc: Maestro of MicroRNAs.
Bui TV, Mendell JT. Bui TV, et al. Genes Cancer. 2010 Jun 1;1(6):568-575. doi: 10.1177/1947601910377491. Genes Cancer. 2010. PMID: 20882107 Free PMC article.
References
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. - PubMed
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. - PubMed
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials