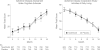Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Dec 16;302(23):2557-64.
doi: 10.1001/jama.2009.1866.
Collaborators, Affiliations
- PMID: 20009055
- PMCID: PMC2902875
- DOI: 10.1001/jama.2009.1866
Randomized Controlled Trial
Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial
Robert C Green et al. JAMA. 2009.
Abstract
Context: Amyloid-beta peptide (Abeta(42)) has been implicated in the pathogenesis of Alzheimer disease (AD). Tarenflurbil, a selective Abeta(42)-lowering agent, demonstrated encouraging results on cognitive and functional outcomes among mildly affected patients in an earlier phase 2 trial.
Objective: To determine the efficacy, safety, and tolerability of tarenflurbil.
Design, setting, and patients: A multicenter, randomized, double-blind, placebo-controlled trial enrolling patients with mild AD was conducted at 133 trial sites in the United States between February 21, 2005, and April 30, 2008. Concomitant treatment with cholinesterase inhibitors or memantine was permitted.
Intervention: Tarenflurbil, 800 mg, or placebo, administered twice a day.
Main outcome measures: Co-primary efficacy end points were the change from baseline to month 18 in total score on the subscale of the Alzheimer Disease Assessment Scale-Cognitive Subscale (ADAS-Cog, 80-point version) and Alzheimer Disease Cooperative Studies-activities of daily living (ADCS-ADL) scale. Additional prespecified slope analyses explored the possibility of disease modification.
Results: Of the 1684 participants randomized, 1649 were included in the analysis, and 1046 completed the trial. Tarenflurbil had no beneficial effect on the co-primary outcomes (difference in change from baseline to month 18 vs placebo, based on least squares means: 0.1 for ADAS-Cog; 95% CI, -0.9 to 1.1; P = .86 and -0.5 for ADCS-ADL; 95% CI, -1.9 to 0.9; P = .48) using an intent-to-treat analysis. No significant differences occurred in the secondary outcomes. The ADAS-Cog score decreased by 7.1 points over 18 months. The tarenflurbil group had a small increase in frequency of dizziness, anemia, and infections.
Conclusion: Tarenflurbil did not slow cognitive decline or the loss of activities of daily living in patients with mild AD.
Trial registration: clinicaltrials.gov Identifier: NCT00105547.
Figures
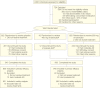
Figure 1
Participant Flow Chart aPatients originally assigned to receive twice daily 400 mg of tarenflurbil were incorporated into the twice daily 800-mg group. bExcluded from the main efficacy analyses using the intent-to-treat population but were included in the safety analyses. Rates of adverse events leading to study discontinuation are based on the intent-to-treat population. The numbers differ from those in the text, which are based on the safety population.
Figure 2
Alzheimer Disease Assessment Cognitive Subscale and Alzheimer Disease Cooperative Studies–Activities of Daily Living Scale Scores by Visit Values represent means using imputed last observation carried forward. Error bars represent 95% CIs.
Comment in
- Late-life dementias: does this unyielding global challenge require a broader view?
Montine TJ, Larson EB. Montine TJ, et al. JAMA. 2009 Dec 16;302(23):2593-4. doi: 10.1001/jama.2009.1863. JAMA. 2009. PMID: 20009062 Free PMC article. No abstract available. - Tarenflurbil: mechanisms and myths.
Sano M. Sano M. Arch Neurol. 2010 Jun;67(6):750-2. doi: 10.1001/archneurol.2010.94. Arch Neurol. 2010. PMID: 20558395 Free PMC article. No abstract available. - Tarenflurbil in patients with mild Alzheimer's disease.
Marder K. Marder K. Curr Neurol Neurosci Rep. 2010 Sep;10(5):336-7. doi: 10.1007/s11910-010-0130-6. Curr Neurol Neurosci Rep. 2010. PMID: 20571933 No abstract available.
Similar articles
- Efficacy and safety of tarenflurbil in mild to moderate Alzheimer's disease: a randomised phase II trial.
Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA; Tarenflurbil Phase II Study investigators. Wilcock GK, et al. Lancet Neurol. 2008 Jun;7(6):483-93. doi: 10.1016/S1474-4422(08)70090-5. Epub 2008 Apr 29. Lancet Neurol. 2008. PMID: 18450517 Clinical Trial. - Efficacy and Safety of Lanabecestat for Treatment of Early and Mild Alzheimer Disease: The AMARANTH and DAYBREAK-ALZ Randomized Clinical Trials.
Wessels AM, Tariot PN, Zimmer JA, Selzler KJ, Bragg SM, Andersen SW, Landry J, Krull JH, Downing AM, Willis BA, Shcherbinin S, Mullen J, Barker P, Schumi J, Shering C, Matthews BR, Stern RA, Vellas B, Cohen S, MacSweeney E, Boada M, Sims JR. Wessels AM, et al. JAMA Neurol. 2020 Feb 1;77(2):199-209. doi: 10.1001/jamaneurol.2019.3988. JAMA Neurol. 2020. PMID: 31764959 Free PMC article. Clinical Trial. - Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer's Disease.
Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, Sur C, Mukai Y, Voss T, Furtek C, Mahoney E, Harper Mozley L, Vandenberghe R, Mo Y, Michelson D. Egan MF, et al. N Engl J Med. 2018 May 3;378(18):1691-1703. doi: 10.1056/NEJMoa1706441. N Engl J Med. 2018. PMID: 29719179 Free PMC article. Clinical Trial. - Memantine for dementia.
McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, Maayan N, Ware J, Debarros J. McShane R, et al. Cochrane Database Syst Rev. 2019 Mar 20;3(3):CD003154. doi: 10.1002/14651858.CD003154.pub6. Cochrane Database Syst Rev. 2019. PMID: 30891742 Free PMC article. - Lack of evidence for the efficacy of memantine in mild Alzheimer disease.
Schneider LS, Dagerman KS, Higgins JP, McShane R. Schneider LS, et al. Arch Neurol. 2011 Aug;68(8):991-8. doi: 10.1001/archneurol.2011.69. Epub 2011 Apr 11. Arch Neurol. 2011. PMID: 21482915 Review.
Cited by
- Peripheral and central effects of γ-secretase inhibition by semagacestat in Alzheimer's disease.
Doody RS, Raman R, Sperling RA, Seimers E, Sethuraman G, Mohs R, Farlow M, Iwatsubo T, Vellas B, Sun X, Ernstrom K, Thomas RG, Aisen PS; Alzheimer’s Disease Cooperative Study. Doody RS, et al. Alzheimers Res Ther. 2015 Jun 10;7(1):36. doi: 10.1186/s13195-015-0121-6. eCollection 2015. Alzheimers Res Ther. 2015. PMID: 26064192 Free PMC article. - Amyloid-β in Alzheimer's disease: Structure, toxicity, distribution, treatment, and prospects.
Yu Y, Yu S, Battaglia G, Tian X. Yu Y, et al. Ibrain. 2024 May 23;10(3):266-289. doi: 10.1002/ibra.12155. eCollection 2024 Fall. Ibrain. 2024. PMID: 39346788 Free PMC article. Review. - Treatment strategies targeting amyloid β-protein.
Schenk D, Basi GS, Pangalos MN. Schenk D, et al. Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a006387. doi: 10.1101/cshperspect.a006387. Cold Spring Harb Perspect Med. 2012. PMID: 22951439 Free PMC article. Review. - Treating Alzheimer's disease by targeting iron.
Nikseresht S, Bush AI, Ayton S. Nikseresht S, et al. Br J Pharmacol. 2019 Sep;176(18):3622-3635. doi: 10.1111/bph.14567. Epub 2019 Feb 11. Br J Pharmacol. 2019. PMID: 30632143 Free PMC article. Review. - γ-Secretase fanning the fire of innate immunity.
Liu C, Nikain C, Li YM. Liu C, et al. Biochem Soc Trans. 2023 Aug 31;51(4):1597-1610. doi: 10.1042/BST20221445. Biochem Soc Trans. 2023. PMID: 37449907 Free PMC article. Review.
References
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. - PubMed
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24-AG027841/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- M01 RR000533/RR/NCRR NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- K24 AG027841/AG/NIA NIH HHS/United States
- M01-RR00533/RR/NCRR NIH HHS/United States
- R01 HG002213/HG/NHGRI NIH HHS/United States
- R01-HG02213/HG/NHGRI NIH HHS/United States
- P30-AG13846/AG/NIA NIH HHS/United States
- DH_/Department of Health/United Kingdom
- P30 AG008051/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous