Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme - PubMed (original) (raw)
Review
Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme
Joost J C Verhoeff et al. BMC Cancer. 2009.
Abstract
Background: The relevance of angiogenesis inhibition in the treatment of glioblastoma multiforme (GBM) should be considered in the unique context of malignant brain tumours. Although patients benefit greatly from reduced cerebral oedema and intracranial pressure, this important clinical improvement on its own may not be considered as an anti-tumour effect.
Discussion: GBM can be roughly separated into an angiogenic component, and an invasive or migratory component. Although this latter component seems inert to anti-angiogenic therapy, it is of major importance for disease progression and survival. We reviewed all relevant literature. Published data support that clinical symptoms are tempered by anti-angiogenic treatment, but that tumour invasion continues. Unfortunately, current imaging modalities are affected by anti-angiogenic treatment too, making it even harder to define tumour margins. To illustrate this we present MRI, biopsy and autopsy specimens from bevacizumab-treated patients.Moreover, while treatment of other tumour types may be improved by combining chemotherapy with anti-angiogenic drugs, inhibiting angiogenesis in GBM may antagonise the efficacy of chemotherapeutic drugs by normalising the blood-brain barrier function.
Summary: Although angiogenesis inhibition is of considerable value for symptom reduction in GBM patients, lack of proof of a true anti-tumour effect raises concerns about the place of this type of therapy in the treatment of GBM.
Figures
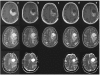
Figure 1
MRI scans of recurrent GBM treated with bevacizumab. MRI scans of a typical patient with recurrent GBM, treated with bevacizumab 10 mg/kg every 3 weeks plus daily temozolomide 50 mg/m2. Top row T1; middle row T2; bottom row ADC (apparent diffusion coefficient. ADC c is lacking). Column (A) scans pre-treatment, showing cystic and tumour component, large midline shift, and large vasogenic oedema. Column (B) 3 days after start, showing reduced contrast enhancement, and slightly reduced midline shift. Column (C) 21 days after start, showing reduced contrast enhancement but a larger size (no progression based on Macdonald criteria), reduced midline shift, and reduced oedema. Column (D) 88 days after start, showing decreased size of tumour and cystic component, stable reduction of contrast enhancement, normalised midline shift, and slight increase of oedema. Column (E) 188 days under treatment, showing increased tumour size and cystic component, increased midline shift, and increased oedema (also in the other hemisphere).
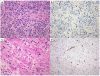
Figure 2
Recurrent GBM: resection and autopsy material, post bevacizumab. (A, B) Recurrent GBM resection material, obtained 6 weeks after last infusion of bevacizumab. Tumour cells co-opt pre-existent vessels with relatively intact BBB (arrows). (A) H&E staining 20×. (B) Glut-1, BBB marker, 20×. (C, D) Recurrent GBM: autopsy was performed 10 weeks after the last infusion of bevacizumab. Near tumour sample shows tumour cells invading along white matter tracks. (C) H&E 20×. (D) Glut-1 BBB marker, 10×.
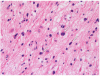
Figure 3
Recurrent GBM: autopsy material, post bevacizumab. GBM cells invade almost the whole brain of this recurrent GBM patient (2C, D). H&E stained autopsy specimen of neocortex of the hemisphere opposite to tumour location, 10 weeks after the last infusion of bevacizumab.
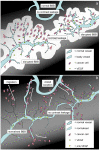
Figure 4
Schematic drawing: pre-treatment and during treatment. Schematic drawing of high-grade glioma, pre-treatment (A), and with anti-VEGF treatment (B). (A) Contrast leakage (white) occurs around leaky tumour vessels enhancing the tumour area on MRI. Capillaries in surrounding tissue are not leaky. (B) Contrast-enhanced area is strongly reduced under anti-VEGF treatment. Tumour cells migrate furtively into the surrounding tissue and co-opt existing vasculature.
Similar articles
- Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms.
Miletic H, Niclou SP, Johansson M, Bjerkvig R. Miletic H, et al. Expert Opin Ther Targets. 2009 Apr;13(4):455-68. doi: 10.1517/14728220902806444. Expert Opin Ther Targets. 2009. PMID: 19335067 Review. - Recurrence pattern in glioblastoma multiforme patients treated with anti-angiogenic chemotherapy.
Tuettenberg J, Grobholz R, Seiz M, Brockmann MA, Lohr F, Wenz F, Vajkoczy P. Tuettenberg J, et al. J Cancer Res Clin Oncol. 2009 Sep;135(9):1239-44. doi: 10.1007/s00432-009-0565-9. Epub 2009 Mar 10. J Cancer Res Clin Oncol. 2009. PMID: 19277712 - Antiangiogenic therapy for high-grade glioma.
Khasraw M, Ameratunga MS, Grant R, Wheeler H, Pavlakis N. Khasraw M, et al. Cochrane Database Syst Rev. 2014 Sep 22;(9):CD008218. doi: 10.1002/14651858.CD008218.pub3. Cochrane Database Syst Rev. 2014. PMID: 25242542 Updated. Review. - Anti-angiogenic therapy in glioma.
Butowski N. Butowski N. Clin Transl Oncol. 2011 May;13(5):294-300. doi: 10.1007/s12094-011-0657-2. Clin Transl Oncol. 2011. PMID: 21596656 Review. - Proteomic analysis predicts anti-angiogenic resistance in recurred glioblastoma.
Jeon H, Byun J, Kang H, Kim K, Lee E, Kim JH, Hong CK, Song SW, Kim YH, Chong S, Kim JH, Nam SJ, Park JE, Lee S. Jeon H, et al. J Transl Med. 2023 Feb 2;21(1):69. doi: 10.1186/s12967-023-03936-8. J Transl Med. 2023. PMID: 36732815 Free PMC article.
Cited by
- Aberrant overexpression of myosin 1b in glioblastoma promotes angiogenesis via VEGF-myc-myosin 1b-Piezo1 axis.
Lv W, Yang F, Ge Z, Xin L, Zhang L, Zhai Y, Liu X, Guo Q, Mao X, Luo P, Zhang L, Jiang X, Zhang Y. Lv W, et al. J Biol Chem. 2024 Sep 20;300(11):107807. doi: 10.1016/j.jbc.2024.107807. Online ahead of print. J Biol Chem. 2024. PMID: 39307302 Free PMC article. - CLEC19A overexpression inhibits tumor cell proliferation/migration and promotes apoptosis concomitant suppression of PI3K/AKT/NF-κB signaling pathway in glioblastoma multiforme.
Mohajerani F, Tehrankhah ZM, Rahmani S, Afsordeh N, Shafiee S, Pourgholami MH, Soltani BM, Sadeghizadeh M. Mohajerani F, et al. BMC Cancer. 2024 Jan 2;24(1):19. doi: 10.1186/s12885-023-11755-9. BMC Cancer. 2024. PMID: 38167030 Free PMC article. - Vascular co-option in resistance to anti-angiogenic therapy.
Ribatti D, Annese T, Tamma R. Ribatti D, et al. Front Oncol. 2023 Dec 11;13:1323350. doi: 10.3389/fonc.2023.1323350. eCollection 2023. Front Oncol. 2023. PMID: 38148844 Free PMC article. Review. - Exploring the dynamic interplay between cancer stem cells and the tumor microenvironment: implications for novel therapeutic strategies.
Li YR, Fang Y, Lyu Z, Zhu Y, Yang L. Li YR, et al. J Transl Med. 2023 Oct 2;21(1):686. doi: 10.1186/s12967-023-04575-9. J Transl Med. 2023. PMID: 37784157 Free PMC article. Review. - The origin of brain malignancies at the blood-brain barrier.
McDonald B, Barth K, Schmidt MHH. McDonald B, et al. Cell Mol Life Sci. 2023 Sep 9;80(10):282. doi: 10.1007/s00018-023-04934-1. Cell Mol Life Sci. 2023. PMID: 37688612 Free PMC article. Review.
References
- Scherer HJ. Structural development in gliomas. Am J Cancer. 1938;34:333–351.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical