Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells - PubMed (original) (raw)
Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells
Joanna J Moser et al. BMC Cancer. 2009.
Abstract
Background: Primary cilia are non-motile sensory cytoplasmic organelles that have been implicated in signal transduction, cell to cell communication, left and right pattern embryonic development, sensation of fluid flow, regulation of calcium levels, mechanosensation, growth factor signaling and cell cycle progression. Defects in the formation and/or function of these structures underlie a variety of human diseases such as Alström, Bardet-Biedl, Joubert, Meckel-Gruber and oral-facial-digital type 1 syndromes. The expression and function of primary cilia in cancer cells has now become a focus of attention but has not been studied in astrocytomas/glioblastomas. To begin to address this issue, we compared the structure and expression of primary cilia in a normal human astrocyte cell line with five human astrocytoma/glioblastoma cell lines.
Methods: Cultured normal human astrocytes and five human astrocytoma/glioblastoma cell lines were examined for primary cilia expression and structure using indirect immunofluorescence and electron microscopy. Monospecific antibodies were used to detect primary cilia and map the relationship between the primary cilia region and sites of endocytosis.
Results: We show that expression of primary cilia in normal astrocytes is cell cycle related and the primary cilium extends through the cell within a unique structure which we show to be a site of endocytosis. Importantly, we document that in each of the five astrocytoma/glioblastoma cell lines fully formed primary cilia are either expressed at a very low level, are completely absent or have aberrant forms, due to incomplete ciliogenesis.
Conclusions: The recent discovery of the importance of primary cilia in a variety of cell functions raises the possibility that this structure may have a role in a variety of cancers. Our finding that the formation of the primary cilium is disrupted in cells derived from astrocytoma/glioblastoma tumors provides the first evidence that altered primary cilium expression and function may be part of some malignant phenotypes. Further, we provide the first evidence that ciliogenesis is not an all or none process; rather defects can arrest this process at various points, particularly at the stage subsequent to basal body association with the plasma membrane.
Figures
Figure 1
Normal human astrocytes contain a single primary cilium. Astrocyte primary cilia were identified extending from the perinuclear region by indirect immunofluorescence (IIF) using antibodies to acetylated (acetyl) tubulin, glu tubulin or adenylyl cyclase III (ACIII) (red) and overlayed with DAPI-stained nuclei (blue). Scale bar = 15 μm.
Figure 2
Astrocyte primary cilia display distinct stages of ciliogenesis. (A) Astrocyte primary cilia ciliogenesis can be divided into five stages as show in the electron micrographs. The double headed arrows indicate that the stages of ciliogenesis displayed by individual astrocytoma/glioblastoma cell lines. At one extreme, T98G glioblastoma/glioblastoma multiforme cells never appear to initiate ciliogenesis, while the other cell lines progress to varying stages. (B) Electron micrographs illustrating representative images from four of the cell lines. 1) Early stage 1 common to U-373 MG, 2),3) and 5) are examples of misshaped or swollen vesicles in U-373 MG, U-138 MG and U-251 MG cells, 4) two centrioles in the same cell arrested at stage 2 in U-251 MG cells, 6) and 7) are examples of unusual ciliary buds in U-251 MG and U-373 MG cells, and 8) show an atypical and rare case of an early axoneme in U-87 MG cells.
Figure 3
The human astrocyte primary cilium resides within a membranous invagination the "cilium-pit" that is involved in endocytosis. Electron micrograph showing a primary cilium extending from the astrocyte cell body into the extracellular matrix. The section was cut obliquely through the basal body (left arrow). The axoneme disappears out of the plane of the section before returning into the focal plane and extends into the extracellular environment. Endocytic vesicles can be seen along the length of the cilium-pit (right arrow, see parallel to shaft of the primary cilium). Scale bar = 250 nm.
Figure 4
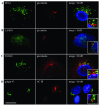
Endocytosis proteins surround the primary cilium. Primary cilia in astrocytes were examined by IIF after co-staining with glu tubulin and AC III antibodies (red). The primary cilium was surrounded by organelles involved in endocytosis including (A) early endosomes as detected using antibodies directed to early endosome antigen 1 (EEA1), (B) late endosomes/lysosomes using antibodies directed to lysosome associated membrane protein 1 (LAMP1), (C) trans Golgi network using antibodies directed to the trans Golgi network 38 (TGN38) and (D) the Golgi complex using antibodies directed to golgin 97 (green). Nuclei were counterstained with DAPI. The inset box is an enlargement of the primary cilia in relation to the EEA1, LAMP1, TGN38 and golgin 97 staining. IIF scale bars = 15 μm.
Figure 5
A primary cilium in the latter stages of ciliogenesis in U-87 MG cells. Electron micrograph showing well formed microtubule doublets (arrows) with normal spacing between doublets in longitudinal section. Scale bar = 100 nm.
Figure 6
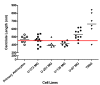
Comparison of centriole length between human astrocytes and five human astrocytoma/glioblastoma cell lines. Centrioles were measured directly on electron micrographs and correlated to scale bars automatically generated by the electron microscope on the same micrograph to arrive at centriole length. Individual points indicate single centrioles examined from separate images. The clusters of centrioles from each individual cell line show the variation of centriole lengths from these separate experiments to form a distribution of centriole lengths. Lines in each distribution represent the mean. The average centriole length based on published values is represented by the red line at 450 nm.
Figure 7
Centrosome length and centriole appendage architecture is abnormal in T98G and U-87 MG cells. Electron micrographs showing abnormal distal appendages (left image) and abnormally long centrioles with sub-distal appendage material distributed along their length (centre and right image). Scale bars = 100 nm.
Similar articles
- Ultrastructural characterization of primary cilia in pathologically characterized human glioblastoma multiforme (GBM) tumors.
Moser JJ, Fritzler MJ, Rattner JB. Moser JJ, et al. BMC Clin Pathol. 2014 Sep 12;14:40. doi: 10.1186/1472-6890-14-40. eCollection 2014. BMC Clin Pathol. 2014. PMID: 25228849 Free PMC article. - LPA signaling is regulated through the primary cilium: a novel target in glioblastoma.
Loskutov YV, Griffin CL, Marinak KM, Bobko A, Margaryan NV, Geldenhuys WJ, Sarkaria JN, Pugacheva EN. Loskutov YV, et al. Oncogene. 2018 Mar;37(11):1457-1471. doi: 10.1038/s41388-017-0049-3. Epub 2018 Jan 11. Oncogene. 2018. PMID: 29321663 Free PMC article. - Repression of GW/P body components and the RNAi microprocessor impacts primary ciliogenesis in human astrocytes.
Moser JJ, Fritzler MJ, Rattner JB. Moser JJ, et al. BMC Cell Biol. 2011 Aug 31;12:37. doi: 10.1186/1471-2121-12-37. BMC Cell Biol. 2011. PMID: 21880135 Free PMC article. - Cell biology of normal and abnormal ciliogenesis in the ciliated epithelium.
Hagiwara H, Ohwada N, Takata K. Hagiwara H, et al. Int Rev Cytol. 2004;234:101-41. doi: 10.1016/S0074-7696(04)34003-9. Int Rev Cytol. 2004. PMID: 15066374 Review. - Assembling a primary cilium.
Kim S, Dynlacht BD. Kim S, et al. Curr Opin Cell Biol. 2013 Aug;25(4):506-11. doi: 10.1016/j.ceb.2013.04.011. Epub 2013 Jun 7. Curr Opin Cell Biol. 2013. PMID: 23747070 Free PMC article. Review.
Cited by
- New software for automated cilia detection in cells (ACDC).
Lauring MC, Zhu T, Luo W, Wu W, Yu F, Toomre D. Lauring MC, et al. Cilia. 2019 Aug 1;8:1. doi: 10.1186/s13630-019-0061-z. eCollection 2019. Cilia. 2019. PMID: 31388414 Free PMC article. - Detection of primary cilia in human glioblastoma.
Sarkisian MR, Siebzehnrubl D, Hoang-Minh L, Deleyrolle L, Silver DJ, Siebzehnrubl FA, Guadiana SM, Srivinasan G, Semple-Rowland S, Harrison JK, Steindler DA, Reynolds BA. Sarkisian MR, et al. J Neurooncol. 2014 Mar;117(1):15-24. doi: 10.1007/s11060-013-1340-y. Epub 2014 Feb 9. J Neurooncol. 2014. PMID: 24510433 Free PMC article. - Mixed signals from the cell's antennae: primary cilia in cancer.
Eguether T, Hahne M. Eguether T, et al. EMBO Rep. 2018 Nov;19(11):e46589. doi: 10.15252/embr.201846589. Epub 2018 Oct 22. EMBO Rep. 2018. PMID: 30348893 Free PMC article. Review. - Primary cilium and glioblastoma.
Álvarez-Satta M, Matheu A. Álvarez-Satta M, et al. Ther Adv Med Oncol. 2018 Oct 3;10:1758835918801169. doi: 10.1177/1758835918801169. eCollection 2018. Ther Adv Med Oncol. 2018. PMID: 30302130 Free PMC article. Review. - The primary cilium as a biomarker in the hypoxic adaptation of bone marrow-derived mesenchymal stromal cells: a role for the secreted frizzled-related proteins.
Proulx-Bonneau S, Annabi B. Proulx-Bonneau S, et al. Biomark Insights. 2011;6:107-18. doi: 10.4137/BMI.S8247. Epub 2011 Sep 29. Biomark Insights. 2011. PMID: 22084569 Free PMC article.
References
- Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Philips M, Teufel A, Kim C, Malik N, Huttner W, Westphal H. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Developmental Biology. 2009;325:24–32. doi: 10.1016/j.ydbio.2008.09.019. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous