Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose-response study in JCR:LA-cp rats - PubMed (original) (raw)
Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose-response study in JCR:LA-cp rats
Jill A Parnell et al. Br J Nutr. 2010 Jun.
Abstract
Prebiotic fibres have been proposed to promote weight loss and lower serum cholesterol; however, the mechanisms are not fully understood. The aim of the present research was to identify possible mechanisms through which prebiotic fibres improve serum lipids. Lean and obese JCR:La-cp rats aged 8 weeks consumed one of three diets supplemented with 0, 10 or 20 % prebiotic fibre for 10 weeks. Rats were anaesthetised and a fasting blood sample was taken for lipid analysis. Real-time PCR was used to determine gene expression for cholesterol and fatty acid regulatory genes in liver tissue. Liver and caecal digesta cholesterol and TAG content were quantified. Both doses of prebiotic fibre lowered serum cholesterol levels by 24 % in the obese hyperlipidaemic rats (P < 0.05). This change was associated with an increase in caecal digesta as well as an up-regulation of genes involved in cholesterol synthesis and bile production. Additionally, there was a 42 % reduction in TAG accumulation in the liver of the obese rats with 10 % prebiotic diet (P < 0.05); however, no change in liver fatty acid synthase (FAS). Prebiotic fibres appear to lower cholesterol levels through increased cholesterol excretion in the form of bile and inhibit the accumulation of TAG in the liver through a mechanism unrelated to FAS. These effects appear to be limited to the obese model and particularly the 10 % dose. The present work is significant as it provides insight into the mechanisms of action for prebiotic fibres on lipid metabolism and furthers the development of dietary treatments for hypercholesterolaemia.
Figures
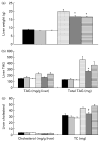
Fig. 1
Liver characteristics for the lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Panel (a) depicts total liver weight. Panel (b) depicts liver TAG content. The series on the left shows TAG content per g of wet liver tissue and the series on the right shows total TAG content of the liver. Total TAG content was calculated by multiplying TAG/g of tissue by total liver weight. For ease of presentation, total liver TAG content was reduced by a factor of 10. Actual values from left to right are 1343·0, 1393·7, 1276·7, 4626·5, 2701·2 and 3719·2 mg. Panel (c) depicts liver cholesterol content. The series on the left shows cholesterol content per gram of wet liver tissue and the series on the right shows total cholesterol (TC) content of the liver. TC content was calculated by multiplying cholesterol per gram of tissue by total liver weight. Data are mean (SE); n 8 except obese high fibre (OHF) group n 7. * Mean values were significantly different from control diet within lean or obese groups. † Mean values were signifi-cantly different between the 10 % and 20 % fibre diets within lean or obese groups as determined by ANOVA with Holm–Sidak adjustment for multiple comparisons or a one-way ANOVA with Bonferroni adjustment where there was a significant diet and group interaction (_P_≤0·05). LC (■), lean control; LF ( ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC ( ), obese control; OF (
), obese control; OF ( ); obese fibre; OHF (▤), obese high fibre.
); obese fibre; OHF (▤), obese high fibre.
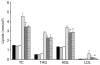
Fig. 2
Serum lipid profiles for lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Results are mean (SE), n 8 except for LDL where some samples were below detection (n 4–6). * Mean values were significantly different from the control diet within the respective lean and obese groups as determined by ANOVA with Holm–Sidak adjustment for multiple comparisons or a one-way ANOVA with Bonferroni adjustment where there was a significant diet by group interaction (_P_≤0·05). LC (■), lean control; LF ( ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC ( ), obese control; OF (
), obese control; OF ( ); obese fibre; OHF (▤), obese high fibre; TC, total cholesterol.
); obese fibre; OHF (▤), obese high fibre; TC, total cholesterol.
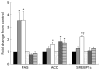
Fig. 3
Liver mRNA levels of genes primarily associated with fatty acid metabolism for lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Results represent the mean fold change (SE) compared to control, n 8 except obese high fibre (OHF) group n 7. * Mean values were significantly different from the control diet within lean and obese groups † Mean values were significantly different between the 10 % and 20 % fibre diets within lean and obese groups as determined by one-way ANOVA with Bonferroni adjustment (_P_≤0·05). LC (■), lean control; LF ( ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC ( ), obese control; OF (
), obese control; OF ( ), obese fibre; OHF (▤), obese high fibre; FAS, fatty acid synthase; ACC, acetyl-CoA carboxy-lase-α; SREBP1c, sterol regulatory element-binding protein 1c.
), obese fibre; OHF (▤), obese high fibre; FAS, fatty acid synthase; ACC, acetyl-CoA carboxy-lase-α; SREBP1c, sterol regulatory element-binding protein 1c.
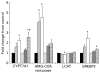
Fig. 4
Liver mRNA levels of genes primarily associated with cholesterol metabolism for lean and obese rats fed control (0 %), fibre (10 %) and high-fibre (20 %) prebiotic diets. Results represent the mean fold change (SE) compared to control, n 8 except obese high fibre (OHF) group n 7. * Mean values were significantly different from the control diet within lean and obese groups. † Mean values were significantly different between the 10 and 20 % fibre diets within lean and obese groups as determined by one-way ANOVA with Bonferroni adjustment (_P_≤0·05). LC (■), lean control; LF ( ), lean fibre; LHF (□), lean high fibre; OC (
), lean fibre; LHF (□), lean high fibre; OC ( ), obese control; OF (
), obese control; OF ( ), obese fibre; OHF (▤), obese high fibre; CYPT7A1, cholesterol 7-α-hydroxylase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LCAT, lecithin:cholesterol acyltransferase; SREBP2, sterol regulatory element-binding protein 2.
), obese fibre; OHF (▤), obese high fibre; CYPT7A1, cholesterol 7-α-hydroxylase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LCAT, lecithin:cholesterol acyltransferase; SREBP2, sterol regulatory element-binding protein 2.
Similar articles
- Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats.
Parnell JA, Reimer RA. Parnell JA, et al. Br J Nutr. 2012 Feb;107(4):601-13. doi: 10.1017/S0007114511003163. Epub 2011 Jul 18. Br J Nutr. 2012. PMID: 21767445 Free PMC article. - Maternal conjugated linoleic acid modulates TAG metabolism in adult rat offspring.
Lavandera J, Gerstner CD, Saín J, Fariña AC, González MA, Bernal CA. Lavandera J, et al. Br J Nutr. 2017 Dec;118(11):906-913. doi: 10.1017/S0007114517003002. Epub 2017 Nov 27. Br J Nutr. 2017. PMID: 29173222 - Effects of inulin-type fructans on lipid metabolism in man and in animal models.
Beylot M. Beylot M. Br J Nutr. 2005 Apr;93 Suppl 1:S163-8. doi: 10.1079/bjn20041339. Br J Nutr. 2005. PMID: 15877890 Review. - Inulin and oligofructose modulate lipid metabolism in animals: review of biochemical events and future prospects.
Delzenne NM, Daubioul C, Neyrinck A, Lasa M, Taper HS. Delzenne NM, et al. Br J Nutr. 2002 May;87 Suppl 2:S255-9. doi: 10.1079/BJNBJN/2002545. Br J Nutr. 2002. PMID: 12088526 Review.
Cited by
- Focus on therapeutic strategies of nonalcoholic Fatty liver disease.
Durazzo M, Belci P, Collo A, Grisoglio E, Bo S. Durazzo M, et al. Int J Hepatol. 2012;2012:464706. doi: 10.1155/2012/464706. Epub 2012 Nov 8. Int J Hepatol. 2012. PMID: 23209914 Free PMC article. - Gut Microbiota and Lifestyle Interventions in NAFLD.
Houghton D, Stewart CJ, Day CP, Trenell M. Houghton D, et al. Int J Mol Sci. 2016 Mar 25;17(4):447. doi: 10.3390/ijms17040447. Int J Mol Sci. 2016. PMID: 27023533 Free PMC article. Review. - Consumption of diets high in prebiotic fiber or protein during growth influences the response to a high fat and sucrose diet in adulthood in rats.
Maurer AD, Eller LK, Hallam MC, Taylor K, Reimer RA. Maurer AD, et al. Nutr Metab (Lond). 2010 Sep 29;7:77. doi: 10.1186/1743-7075-7-77. Nutr Metab (Lond). 2010. PMID: 20920272 Free PMC article. - The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity.
Cerdó T, García-Santos JA, G Bermúdez M, Campoy C. Cerdó T, et al. Nutrients. 2019 Mar 15;11(3):635. doi: 10.3390/nu11030635. Nutrients. 2019. PMID: 30875987 Free PMC article. Review. - The Prebiotic Inulin Aggravates Accelerated Atherosclerosis in Hypercholesterolemic APOE*3-Leiden Mice.
Hoving LR, de Vries MR, de Jong RCM, Katiraei S, Pronk A, Quax PHA, van Harmelen V, Willems van Dijk K. Hoving LR, et al. Nutrients. 2018 Feb 3;10(2):172. doi: 10.3390/nu10020172. Nutrients. 2018. PMID: 29401645 Free PMC article.
References
- Kissebah AH, Freedman DS, Peiris AN. Health risks of obesity. Med Clin North Am. 1989;73:111–138. - PubMed
- Levrat M-A, Favier M-L, Moundras C, et al. Role of dietary propionic acid and bile acid excretion in the hypo-cholesterolemic effects of oligosaccharides in rats. J Nutr. 1994;124:531–538. - PubMed
- Cani PD, Neyrinck AM, Maton N, et al. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res. 2005;13:1000–1007. - PubMed
- Urias-Silvas JE, Cani PD, Delmee E, et al. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br J Nutr. 2008;99:254–261. - PubMed
- Rault-Nania MH, Gueux E, Demougeot C, et al. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. 2006;96:840–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous