Analysis of 8-oxo-7,8-dihydro-2'-deoxyguanosine by ultra high pressure liquid chromatography-heat assisted electrospray ionization-tandem mass spectrometry - PubMed (original) (raw)
Analysis of 8-oxo-7,8-dihydro-2'-deoxyguanosine by ultra high pressure liquid chromatography-heat assisted electrospray ionization-tandem mass spectrometry
Gunnar Boysen et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2010.
Abstract
Increased amounts of reactive oxygen species (ROS), generally termed oxidative stress, are frequently hypothesized to be causally associated with many diseases. Analyses of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dG) in DNA and urine are widely used biomarkers for oxidative stress. Over the years it became clear that analysis of 8-oxo-dG in DNA is challenging due to artifactual formation during sample work up. The present study demonstrates that 8-oxo-dG can be measured reliably and accurately when appropriate precautions are taken. First, the presence of an antioxidant, metal chelator, or free radical trapping agent during sample preparation improves reproducibility. Second, sample enrichment by HPLC fraction collection was used to optimize sensitivity. Third, heat assisted electrospray ionization (HESI) eliminated potential interferences and improved assay performance and sensitivity. Subsequently, the UPLC-HESI-MS/MS method was applied to show the biphasic dose response of 8-oxo-dG in H(2)O(2)-treated HeLa cells. Application of this method to human lymphocyte DNA (n=156) gave a mean+/-SD endogenous amount of 1.57+/-0.88 adducts per 10(6) dG, a value that is in agreement with the suggested amount previously estimated by European Standard Committee on Oxidative DNA Damage (ESCODD) and others. These results suggest that the present method is well suited for application to molecular toxicology and epidemiology studies investigating the role of oxidative stress.
Published by Elsevier B.V.
Figures
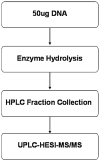
Fig. 1
Schematic outline of analysis protocol.
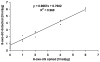
Fig. 2
Control rat liver DNA spiked with authentic 8-oxo-dG (negative control, n = 9; positive control, n = 3) was analyzed for 8-oxo-dG by UPLC–HESI–MS/MS as described in materials and methods.
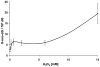
Fig. 3
Biphasic dose response in H2O2-treated HeLa cells (n = 6). HeLa cells were treated with different concentrations of H2O2 for 15 min and amounts of 8-oxo-dG in DNA were measured by UPLC–HESI–MS/MS as described in materials and method.
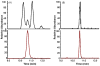
Fig. 4
Extracted ion chromatogram of 8-oxo-dG and [15N5]8-oxo-dG in rat liver DNA analyzed by (A) capillary LC–MS/MS or (B) UPLC–HESI–MS/MS. Shown are the transitions of m/z 284.1 → 168.0 and m/z 289.1 → 173.0 for 8-oxo-dG (upper traces) and [15N5]8-oxo-dG (lower traces), respectively.
Fig. 5
Endogenous 8-oxo-dG in human peripheral blood lymphocytes and snap frozen rat liver DNA measured by UPLC–HESI–MS/MS. The mean±SD amount of 8-oxo-dG in human lymphocytes was 1.57±0.88 8-oxo-dG/106 dG (n = 156).
Similar articles
- Analysis of 7,8-dihydro-8-oxo-2'-deoxyguanosine in cellular DNA during oxidative stress.
Mangal D, Vudathala D, Park JH, Lee SH, Penning TM, Blair IA. Mangal D, et al. Chem Res Toxicol. 2009 May;22(5):788-97. doi: 10.1021/tx800343c. Chem Res Toxicol. 2009. PMID: 19309085 Free PMC article. - Simultaneous determination of 8-oxo-2'-deoxyguanosine and 8-oxo-2'-deoxyadenosine in human retinal DNA by liquid chromatography nanoelectrospray-tandem mass spectrometry.
Ma B, Jing M, Villalta PW, Kapphahn RJ, Montezuma SR, Ferrington DA, Stepanov I. Ma B, et al. Sci Rep. 2016 Mar 16;6:22375. doi: 10.1038/srep22375. Sci Rep. 2016. PMID: 26979577 Free PMC article. - Quantitative analysis of the oxidative DNA lesion, 2,2-diamino-4-(2-deoxy-beta-D-erythro-pentofuranosyl)amino]-5(2H)-oxazolone (oxazolone), in vitro and in vivo by isotope dilution-capillary HPLC-ESI-MS/MS.
Matter B, Malejka-Giganti D, Csallany AS, Tretyakova N. Matter B, et al. Nucleic Acids Res. 2006;34(19):5449-60. doi: 10.1093/nar/gkl596. Epub 2006 Oct 4. Nucleic Acids Res. 2006. PMID: 17020926 Free PMC article. - 8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis.
Valavanidis A, Vlachogianni T, Fiotakis C. Valavanidis A, et al. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009 Apr;27(2):120-39. doi: 10.1080/10590500902885684. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009. PMID: 19412858 Review. - The Influence of 2'-Deoxyguanosine Lesions on the Electronic Properties of OXOG:::C Base Pairs in Ds-DNA: A Comparative Analysis of Theoretical Studies.
Karwowski BT. Karwowski BT. Molecules. 2024 Aug 8;29(16):3756. doi: 10.3390/molecules29163756. Molecules. 2024. PMID: 39202837 Free PMC article. Review.
Cited by
- Current and Future Methodology for Quantitation and Site-Specific Mapping the Location of DNA Adducts.
Boysen G, Nookaew I. Boysen G, et al. Toxics. 2022 Jan 19;10(2):45. doi: 10.3390/toxics10020045. Toxics. 2022. PMID: 35202232 Free PMC article. - Fluorescence Imaging of Mitochondrial DNA Base Excision Repair Reveals Dynamics of Oxidative Stress Responses.
Jun YW, Albarran E, Wilson DL, Ding J, Kool ET. Jun YW, et al. Angew Chem Int Ed Engl. 2022 Feb 1;61(6):e202111829. doi: 10.1002/anie.202111829. Epub 2021 Dec 22. Angew Chem Int Ed Engl. 2022. PMID: 34851014 Free PMC article. - Interactions of Mitochondrial Transcription Factor A with DNA Damage: Mechanistic Insights and Functional Implications.
Chew K, Zhao L. Chew K, et al. Genes (Basel). 2021 Aug 15;12(8):1246. doi: 10.3390/genes12081246. Genes (Basel). 2021. PMID: 34440420 Free PMC article. Review. - Mitochondrial DNA Damage: Prevalence, Biological Consequence, and Emerging Pathways.
Zhao L, Sumberaz P. Zhao L, et al. Chem Res Toxicol. 2020 Oct 19;33(10):2491-2502. doi: 10.1021/acs.chemrestox.0c00083. Epub 2020 Jun 18. Chem Res Toxicol. 2020. PMID: 32486637 Free PMC article. Review. - Quantitation of Lipid Peroxidation Product DNA Adducts in Human Prostate by Tandem Mass Spectrometry: A Method That Mitigates Artifacts.
Chen H, Krishnamachari S, Guo J, Yao L, Murugan P, Weight CJ, Turesky RJ. Chen H, et al. Chem Res Toxicol. 2019 Sep 16;32(9):1850-1862. doi: 10.1021/acs.chemrestox.9b00181. Epub 2019 Aug 16. Chem Res Toxicol. 2019. PMID: 31361128 Free PMC article.
References
- Ishii N. Cornea. 2007;26:S3. - PubMed
- ESCODD. Carcinogenesis. 2002;23:2129. - PubMed
- ESCODD. Free Radic Biol Med. 2003;34:1089. - PubMed
- Ravanat JL, Turesky RJ, Gremaud E, Trudel LJ, Stadler RH. Chem Res Toxicol. 1995;8:1039. - PubMed
- Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Free Radic Biol Med. 2000;28:13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES10126/ES/NIEHS NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- P42-ES05948/ES/NIEHS NIH HHS/United States
- R21 ES019684/ES/NIEHS NIH HHS/United States
- R01 ES012689/ES/NIEHS NIH HHS/United States
- P42 ES005948/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources