Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning - PubMed (original) (raw)
Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning
Trung H M Pham et al. J Exp Med. 2010.
Abstract
Lymphocyte egress from lymph nodes (LNs) is dependent on sphingosine-1-phosphate (S1P), but the cellular source of this S1P is not defined. We generated mice that expressed Cre from the lymphatic vessel endothelial hyaluronan receptor 1 (Lyve-1) locus and that showed efficient recombination of loxP-flanked genes in lymphatic endothelium. We report that mice with Lyve-1 CRE-mediated ablation of sphingosine kinase (Sphk) 1 and lacking Sphk2 have a loss of S1P in lymph while maintaining normal plasma S1P. In Lyve-1 Cre+ Sphk-deficient mice, lymphocyte egress from LNs and Peyer's patches is blocked. Treatment with pertussis toxin to overcome Galphai-mediated retention signals restores lymphocyte egress. Furthermore, in the absence of lymphatic Sphks, the initial lymphatic vessels in nonlymphoid tissues show an irregular morphology and a less organized vascular endothelial cadherin distribution at cell-cell junctions. Our data provide evidence that lymphatic endothelial cells are an in vivo source of S1P required for lymphocyte egress from LNs and Peyer's patches, and suggest a role for S1P in lymphatic vessel maturation.
Figures
Figure 1.
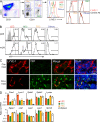
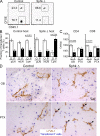
Efficiency of Lyve-1 CRE-mediated gene deletion and S1P metabolic enzyme expression in LECs. (A) Isolation and identification of LECs. LNs were minced and digested as detailed in Materials and methods. CD31 and gp38 expression allows separation of LECs from other CD45− cells: FRCs, BECs, and double-negative stromal cells (Others). (left plot) LYVE-1 on indicated populations; (right plots) Control stains for each cell type. (B) Flow cytometric analysis of YFP in cells isolated from LNs of Lyve-1 Cre+ Rosa26-YFP reporter mice (percentages are shown). The four cell populations are gated according to the scheme in A. Peripheral LNs (pLN; axillary, brachial, and inguinal nodes) and mesenteric LNs (mLN) are shown. The shaded histogram represents Cre-negative cells. (C) Immunofluorescence analysis of YFP in LNs of Lyve-1 Cre+ or Rosa26-YFP mice that had been reconstituted with wild-type BM. Fixed LN sections were stained as indicated. Data in A–C are representative of at least three experiments with one to two mice of each type per experiment. (D) Quantitative RT-PCR analysis of S1P metabolic genes in cells sorted from a pool of peripheral and mesenteric LNs using the scheme in A and from spleen tissue or splenic B cells. Data are representative of three separate sorts with 10–15 mice in each sort, with each gene expression measured at least twice. Bars represent means.
Figure 2.
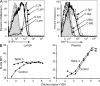
Ablation of lymph S1P by conditional deletion of Sphk1 in _Sphk2_-deficient mice. (A) Flow cytometric analysis showing S1P1 on CD4+ CD62Lhi T cells from the indicated circulatory fluids and tissues. Δ indicates Lyve-1 Cre+ Sphk1f/− or f/f Sphk2−/− mice; C indicates littermate control. The shaded histograms show staining with control antibody of cells from LN and blood. Bld, blood; Lym, lymph; Spl, spleen. (B) Measurement of S1P level by bioassay. Lymph fluid and plasma samples were prepared (see Materials and methods) and titrated onto WEHI231 cells expressing FLAG-S1P1. The x axis shows dilution of the samples. The y axis indicates mean fluorescence intensity (MFI) of FLAG antibody staining. Data are representative of at least five experiments with one to two mice each.
Figure 3.
Lack of contribution of myeloid cells to lymph S1P. (A) Flow cytometric analysis of enzyme-digested LN cells to detect CD11b and CD11c. (B) LYVE-1 or control antibody staining of the six cell populations shown in A. (C) The proportion of each subpopulation replaced by donor-derived cells in lethally irradiated CD45.2+ _Lyve-1 Cre+ Sphk_-deficient mice reconstituted with wild-type CD45.1+ BM. Numbers refer to the percentage of cells in the indicated gates. (D) S1P1 on CD4+ CD62Lhi T cells from the lymph and LNs of mice that had been reconstituted with wild-type BM as in C. Sphk Δ indicates the Lyve-1 Cre+ Sphk-deficient host; C indicates the control host. The shaded histogram shows staining of LN cells from an FTY720-treated mouse. (E) Bioassay measurement of S1P in lymph and plasma from the chimeric mice. Data in A–E are representative of three experiments with three mice. MFI, mean fluorescence intensity.
Figure 4.
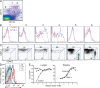
Impaired lymphocyte egress in _Lyve-1 Cre Sphk_-deficient mice. (A–E) Cell numbers in the indicated fluids and tissues in _Lyve-1 Cre+ Sphk_-deficient and control mice. The LN count was from a pool of two axillary, brachial, and inguinal LNs. Enumerated CD4+, CD8+, and CD19+ cells were CD62Lhi. Points indicate data from individual mice, and white (control mice) and black (_Sphk_-deficient mice) bars represent means. (F) S1P1 on CD4+ CD62Lhi T cells from the indicated tissues. Shaded histograms show staining with control antibody. The vertical dashed lines mark the peak S1P1 intensity in the LN sample to allow comparison. Data in A–F are representative of at least three experiments with one to two mice per experiment. (G and H) Immunohistochemial analysis of LNs (G) and Peyer's patches (H) stained for LYVE-1 (brown) and CD3 (blue) or B220 (blue).
Figure 5.
PTX treatment facilitates lymphocyte egress and localization in cortical sinuses in _Lyve-1 Cre+ Sphk_-deficient mice. (A–C) Splenocytes were treated with either PTX or OB and cotransferred into recipient hosts. 22 h later, transferred cell numbers were determined in the lymph and LNs of Lyve-1 Cre+ _Sphk_-deficient (Sphk Δ) and control hosts. (A) Flow cytometric analysis of transferred T cells present in the lymph. PTX-treated cells were CFSE labeled. Numbers refer to the percentage of cells in the indicated gates. (B) Frequency of transferred OB (O)- and PTX (P)-treated cells in peripheral LNs (pLN), mesenteric LNs (mLN), and the lymph (Lym). (C) Total numbers of transferred CD4 and CD8 T cells in the lymph of control and _Sphk_-deficient recipients from the same experiments as in B. In B and C, points indicate data from individual mice, and bars indicate means. (D) Distribution of transferred OB- and PTX-treated cells with respect to LN cortical sinuses. Purified T cells were treated and cotransferred into control or Sphk Δ hosts as in A–C. Sections were stained for LYVE-1 (brown) and transferred T cells (blue). Several transferred cells located within sinuses of the _Sphk_-deficient recipient are marked by arrows. Data in A–D are representative of at least three experiments with one to two mice per experiment. C, control; Δ, Sphk deficient.
Figure 6.
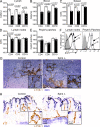
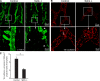
Lymphatic Sphk expression is required for normal lymphatic vessel maturation. (A) Confocal images showing lymphatic vessels stained with antibody against LYVE-1 in whole mount of mouse trachea. Arrowheads point to the jagged appearance of the lymphatic vessels in _Lyve-1 Cre+ Sphk_-deficient mice. (B) Confocal images showing the button-like pattern of VE-cadherin at endothelial cell–cell junctions of the diaphragm initial lymphatics. Arrowheads mark VE-cadherin+ buttons in the control and a corresponding junctional region in the _Lyve-1 Cre+ Sphk_-deficient mice. White squares (top) indicate enlarged regions (bottom). Data are representative of six experiments (n = 6 mice). (C) VE-cadherin buttons per 1,000-µm2 projected area of lymphatic for littermate control and lymphatic Sphk-deficient trachea (n = 3 mice per group). Bars show means ± SE. *, P < 0.05 (Student's t test).
Similar articles
- Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells.
Mendoza A, Fang V, Chen C, Serasinghe M, Verma A, Muller J, Chaluvadi VS, Dustin ML, Hla T, Elemento O, Chipuk JE, Schwab SR. Mendoza A, et al. Nature. 2017 Jun 1;546(7656):158-161. doi: 10.1038/nature22352. Epub 2017 May 24. Nature. 2017. PMID: 28538737 Free PMC article. - Sphingosine 1-phosphate regulates the egress of IgA plasmablasts from Peyer's patches for intestinal IgA responses.
Gohda M, Kunisawa J, Miura F, Kagiyama Y, Kurashima Y, Higuchi M, Ishikawa I, Ogahara I, Kiyono H. Gohda M, et al. J Immunol. 2008 Apr 15;180(8):5335-43. doi: 10.4049/jimmunol.180.8.5335. J Immunol. 2008. PMID: 18390715 - Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells.
Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Tauseef M, et al. Circ Res. 2008 Nov 7;103(10):1164-72. doi: 10.1161/01.RES.0000338501.84810.51. Epub 2008 Oct 10. Circ Res. 2008. PMID: 18849324 Free PMC article. - Sphingosine kinase signalling in immune cells: potential as novel therapeutic targets.
Melendez AJ. Melendez AJ. Biochim Biophys Acta. 2008 Jan;1784(1):66-75. doi: 10.1016/j.bbapap.2007.07.013. Epub 2007 Aug 14. Biochim Biophys Acta. 2008. PMID: 17913601 Review. - Immune regulation by sphingosine 1-phosphate and its receptors.
Bode C, Gräler MH. Bode C, et al. Arch Immunol Ther Exp (Warsz). 2012 Feb;60(1):3-12. doi: 10.1007/s00005-011-0159-5. Epub 2011 Dec 8. Arch Immunol Ther Exp (Warsz). 2012. PMID: 22159476 Review.
Cited by
- Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy.
Proia RL, Hla T. Proia RL, et al. J Clin Invest. 2015 Apr;125(4):1379-87. doi: 10.1172/JCI76369. Epub 2015 Apr 1. J Clin Invest. 2015. PMID: 25831442 Free PMC article. Review. - Sphingolipid metabolism in T cell responses after allogeneic hematopoietic cell transplantation.
Tian L, Ogretmen B, Chung BY, Yu XZ. Tian L, et al. Front Immunol. 2022 Aug 16;13:904823. doi: 10.3389/fimmu.2022.904823. eCollection 2022. Front Immunol. 2022. PMID: 36052066 Free PMC article. Review. - In Sickness and in Health: The Immunological Roles of the Lymphatic System.
Johnson LA. Johnson LA. Int J Mol Sci. 2021 Apr 24;22(9):4458. doi: 10.3390/ijms22094458. Int J Mol Sci. 2021. PMID: 33923289 Free PMC article. Review. - Targeting the SphK-S1P-SIPR Pathway as a Potential Therapeutic Approach for COVID-19.
McGowan EM, Haddadi N, Nassif NT, Lin Y. McGowan EM, et al. Int J Mol Sci. 2020 Sep 29;21(19):7189. doi: 10.3390/ijms21197189. Int J Mol Sci. 2020. PMID: 33003377 Free PMC article. Review. - HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes.
Girard JP, Moussion C, Förster R. Girard JP, et al. Nat Rev Immunol. 2012 Nov;12(11):762-73. doi: 10.1038/nri3298. Epub 2012 Sep 28. Nat Rev Immunol. 2012. PMID: 23018291 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL024136/HL/NHLBI NIH HHS/United States
- R01 HL059157/HL/NHLBI NIH HHS/United States
- R01 HL065590/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous