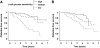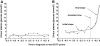Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset - PubMed (original) (raw)
Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset
Ele Ferrannini et al. Diabetes. 2010 Mar.
Abstract
Objective: Relatives of type 1 diabetic patients are at enhanced risk of developing diabetes. We investigated the mode of onset of hyperglycemia and how insulin sensitivity and beta-cell function contribute to the progression to the disease.
Research design and methods: In 328 islet cell autoantibody-positive, nondiabetic relatives from the observational arms of the Diabetes Prevention Trial-1 Study (median age 11 years [interquartile range 8], sequential OGTTs (2,143 in total) were performed at baseline, every 6 months, and 2.7 years [2.7] later, when 115 subjects became diabetic. Beta-cell glucose sensitivity (slope of the insulin-secretion/plasma glucose dose-response function) and insulin sensitivity were obtained by mathematical modeling of the OGTT glucose/C-peptide responses.
Results: In progressors, baseline insulin sensitivity, fasting insulin secretion, and total postglucose insulin output were similar to those of nonprogressors, whereas beta-cell glucose sensitivity was impaired (median 48 pmol/min per m2 per mmol/l [interquartile range 36] vs. 87 pmol/min per m2 per mmol/l [67]; P < 0.0001) and predicted incident diabetes (P < 0.0001) independently of sex, age, BMI, and clinical risk. In progressors, 2-h glucose levels changed little until 0.78 years before diagnosis, when they started to rise rapidly (approximately 13 mmol x l(-1) x year(-1)); glucose sensitivity began to decline significantly (P < 0.0001) earlier (1.45 years before diagnosis) than the plasma glucose surge. During this anticipation phase, both insulin secretion and insulin sensitivity were essentially stable.
Conclusions: In high-risk relatives, beta-cell glucose sensitivity is impaired and is a strong predictor of diabetes progression. The time trajectories of plasma glucose are frequently biphasic, with a slow linear increase followed by a rapid surge, and are anticipated by a further deterioration of beta-cell glucose sensitivity.
Figures
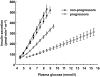
FIG. 1.
Dose-response curve of insulin secretion rates vs. plasma glucose levels during the OGTT in 280 subjects with normal glucose tolerance according to whether (progressors) or not (nonprogressors) they developed diabetes. Data are means ± SEM. The mean slope of the dose response is β-cell glucose sensitivity. Full lines, baseline data; dotted lines, data at follow-up.
FIG. 2.
Kaplan-Meier plots of diabetes-free survival in 280 subjects with normal glucose tolerance at baseline according to tertile of baseline β-cell glucose sensitivity (log-rank χ2 = 25.5, P < 0.0001, and 13.2, P = 0.0003, in female [_A_] and male [_B_] subjects, respectively). The risk ratio of subjects in the bottom tertile of β-cell glucose sensitivity (dotted line) is 2.40 (95% CI 1.76–3.30), P < 0.0001 vs. the top tertile, adjusted for sex, age, and BMI.
FIG. 3.
Example of a monophasic (A) (subject YW3599) and a biphasic (B) (subject HR2920) time course of 2-h plasma glucose concentrations; note the change in slope at transition time in the biphasic time pattern.
FIG. 4.
Half-yearly averages of 2-h plasma glucose concentrations (left panel) and individual fits of their time series (right panel) in nonprogressors and progressors. The thick lines are the mean of the individual fitting functions.
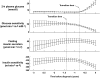
FIG. 5.
Time course of 2-h plasma glucose concentrations, glucose sensitivity, fasting insulin secretion, and insulin sensitivity in the progressor group. Time series are synchronized on the transition time of 2-h plasma glucose levels.
Similar articles
- Impaired beta cell glucose sensitivity and whole-body insulin sensitivity as predictors of hyperglycaemia in non-diabetic subjects.
Walker M, Mari A, Jayapaul MK, Bennett SM, Ferrannini E. Walker M, et al. Diabetologia. 2005 Dec;48(12):2470-6. doi: 10.1007/s00125-005-0004-7. Epub 2005 Nov 1. Diabetologia. 2005. PMID: 16261308 - Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span.
RISE Consortium. RISE Consortium. Diabetes Care. 2014;37(3):780-8. doi: 10.2337/dc13-1879. Epub 2013 Nov 5. Diabetes Care. 2014. PMID: 24194506 Free PMC article. Clinical Trial. - Goals of treatment for type 2 diabetes: beta-cell preservation for glycemic control.
Marchetti P, Lupi R, Del Guerra S, Bugliani M, D'Aleo V, Occhipinti M, Boggi U, Marselli L, Masini M. Marchetti P, et al. Diabetes Care. 2009 Nov;32 Suppl 2(Suppl 2):S178-83. doi: 10.2337/dc09-S306. Diabetes Care. 2009. PMID: 19875548 Free PMC article. Review. No abstract available. - C-peptide in the natural history of type 1 diabetes.
Palmer JP. Palmer JP. Diabetes Metab Res Rev. 2009 May;25(4):325-8. doi: 10.1002/dmrr.943. Diabetes Metab Res Rev. 2009. PMID: 19267337 Free PMC article. Review.
Cited by
- Progressive erosion of β-cell function precedes the onset of hyperglycemia in the NOD mouse model of type 1 diabetes.
Ize-Ludlow D, Lightfoot YL, Parker M, Xue S, Wasserfall C, Haller MJ, Schatz D, Becker DJ, Atkinson MA, Mathews CE. Ize-Ludlow D, et al. Diabetes. 2011 Aug;60(8):2086-91. doi: 10.2337/db11-0373. Epub 2011 Jun 9. Diabetes. 2011. PMID: 21659497 Free PMC article. - Deoxyhypusine synthase promotes differentiation and proliferation of T helper type 1 (Th1) cells in autoimmune diabetes.
Colvin SC, Maier B, Morris DL, Tersey SA, Mirmira RG. Colvin SC, et al. J Biol Chem. 2013 Dec 20;288(51):36226-35. doi: 10.1074/jbc.M113.473942. Epub 2013 Nov 6. J Biol Chem. 2013. PMID: 24196968 Free PMC article. - Exocytosis Protein DOC2B as a Biomarker of Type 1 Diabetes.
Aslamy A, Oh E, Ahn M, Moin ASM, Chang M, Duncan M, Hacker-Stratton J, El-Shahawy M, Kandeel F, DiMeglio LA, Thurmond DC. Aslamy A, et al. J Clin Endocrinol Metab. 2018 May 1;103(5):1966-1976. doi: 10.1210/jc.2017-02492. J Clin Endocrinol Metab. 2018. PMID: 29506054 Free PMC article. - Early prediction of autoimmune (type 1) diabetes.
Regnell SE, Lernmark Å. Regnell SE, et al. Diabetologia. 2017 Aug;60(8):1370-1381. doi: 10.1007/s00125-017-4308-1. Epub 2017 May 26. Diabetologia. 2017. PMID: 28550517 Free PMC article. Review. - A little help from residual β cells has long-lasting clinical benefits.
Lam A, Dayan C, Herold KC. Lam A, et al. J Clin Invest. 2021 Feb 1;131(3):e143683. doi: 10.1172/JCI143683. J Clin Invest. 2021. PMID: 33529163 Free PMC article.
References
- Gorsuch AN, Spencer KM, Lister J, McNally JM, Dean BM, Bottazzo GF, Cudworth AG: Evidence for a long prediabetic period in type I (insulin-dependent) diabetes mellitus. Lancet 1981; 2: 1363– 1365 - PubMed
- Eisenbarth GS: Prediction of type 1 diabetes: the natural history of the prediabetic period. Adv Exp Med Biol 2004; 552: 268– 290 - PubMed
- Bonifacio E, Bingley PJ, Shattock M, Dean BM, Dunger D, Gale EA, Bottazzo GF: Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet 1990; 335: 147– 149 - PubMed
- Riley WJ, Maclaren NK, Krischer J, Spillar RP, Silverstein JH, Schatz DA, Schwartz S, Malone J, Shah S, Vadheim C, et al. : A prospective study of the development of diabetes in relatives of patients with insulin-dependent diabetes. N Engl J Med 1990; 323: 1167– 1172 - PubMed
- Wasserfall CH, Atkinson MA: Autoantibody markers for the diagnosis and prediction of type 1 diabetes. Autoimmun Rev 2006; 5: 424– 428 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical