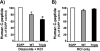Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion - PubMed (original) (raw)
Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion
Zhanxiang Wang et al. J Biol Chem. 2010.
Abstract
Cdc42 cycling through GTP/GDP states is critical for its function in the second/granule mobilization phase of insulin granule exocytosis in pancreatic islet beta cells, although the identities of the Cdc42 cycling proteins involved remain incomplete. Using a tandem affinity purification-based mass spectrometry screen for Cdc42 cycling factors in beta cells, RhoGDI was identified. RNA interference-mediated depletion of RhoGDI from isolated islets selectively amplified the second phase of insulin release, consistent with the role of RhoGDI as a Cdc42 cycling factor. Replenishment of RhoGDI to RNA interference-depleted cells normalized secretion, confirming the action of RhoGDI to be that of a negative regulator of Cdc42 activation. Given that RhoGDI also regulates Rac1 activation in beta cells, and that Rac1 activation occurs in a Cdc42-dependent manner, the question as to how the beta cell utilized RhoGDI for differential Cdc42 and Rac1 cycling was explored. Co-immunoprecipitation was used to determine that RhoGDI-Cdc42 complexes dissociated upon stimulation of beta cells with glucose for 3 min, correlating with the timing of glucose-induced Cdc42 activation and the onset of RhoGDI tyrosine phosphorylation. Glucose-induced disruption of RhoGDI-Rac1 complexes occurred subsequent to this, coincident with Rac1 activation, which followed the onset of RhoGDI serine phosphorylation. RhoGDI-Cdc42 complex dissociation was blocked by mutation of RhoGDI residue Tyr-156, whereas RhoGDI-Rac1 dissociation was blocked by RhoGDI mutations Y156F and S101A/S174A. Finally, expression of a triple Y156F/S101A/S174A-RhoGDI mutant specifically inhibited only the second/granule mobilization phase of glucose-stimulated insulin secretion, overall supporting the integration of RhoGDI into the activation cycling mechanism of glucose-responsive small GTPases.
Figures
FIGURE 1.
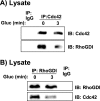
RhoGDI interacts with Cdc42 in MIN6 beta cells. A, MIN6 cells were transduced with TAP-Cdc42 or control TAP-CAT adenoviruses (multiplicity of infection = 100). Lysates were subsequently prepared (lanes 1 and 2) and TAP-Cdc42-purified over SBP and CBP columns to generate eluate (lane 3), which were subjected to 12% SDS-PAGE to resolve proteins for immunoblotting (IB) using Cdc42 antibody. B, lysates from unstimulated MIN6 β-cells were immunoprecipitated with Cdc42 or nonspecific IgG antibodies and precipitates resolved on 12% SDS-PAGE for immunodetection of RhoGDI and Cdc42. C, immunodetection of Cdc42 and RhoGDI proteins in human or mouse islets (100 islets per lane) and MIN6 beta cells (30 μg lysate protein) resolved by 12% SDS-PAGE. D, RNA (1 μg) from MIN6 cells and mouse islets was reverse-transcribed with TaqMan, and 1% of the complementary DNA was used for RT-PCR using primers to detect three RhoGDI isoforms (α, β, and γ). The products were separated by electrophoresis in 2% agarose and visualized with ethidium bromide. E, MIN6 cells were preincubated in glucose-free MKRBB for 2 h prior to subcellular fractionation into plasma membrane (PM), cytosol (Cyto), and storage granule (SG) fractions. Fraction proteins (20 μg) were subjected to 12% SDS-PAGE for immunoblotting (IB) Cav-1, caveolin-1. F, Cdc42-RhoGDI complexes were co-immunoprecipitated from cytosolic fractions. Each data panel is representative of three independent experiments.
FIGURE 2.
Glucose-induced transient dissociation of cytosolic Cdc42-RhoGDI complexes. MIN6 cells were incubated in MKRBB for 2 h and left unstimulated or were stimulated with 20 m
m
glucose (Gluc) for 3 min. A, detergent cell lysates were prepared for IP with anti-Cdc42 or control IgG antibodies. Immunoprecipitates were subjected to 12% SDS-PAGE for subsequent immunoblotting (IB). B, lysates prepared from unstimulated or glucose-stimulated (3 min) MIN6 cells were used for anti-RhoGDI immunoprecipitation; proteins were resolved by 12% SDS-PAGE for immunoblotting. Data are representative of at least three independent experiments.
FIGURE 3.
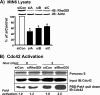
Depletion of RhoGDI enhances glucose-induced Cdc42 activation. A, MIN6 cells were transfected with three different commercially available RhoGDIα siRNA (siA, siB, and siC) or negative control (siCon) oligonucleotides using Lipofectamine 2000 as described under “Experimental Procedures.” After 48 h of incubation, whole cell detergent lysates were prepared and subjected to 12% SDS-PAGE for immunoblotting with anti-RhoGDI and anti-actin (loading control) antibodies. The bar graph shows the quantitation by optical density scanning of RhoGDI depletion compared with siCon in three independent experiments; *, p < 0.05. B, detergent cell lysates were prepared from MIN6 cells transfected with siCon or the siA oligonucleotides (siRhoGDI), left unstimulated or glucose (Gluc)-stimulated for 3 min, for immediate use in the GST-Pak1-PBD interaction assay. Eluted proteins were resolved on 12% SDS-PAGE for subsequent immunoblotting with mouse anti-Cdc42 antibody. Ponceau S staining served as an indicator of GST-Pak1-PBD loading, and immunoblotting of input lysate shows equal Cdc42 expression under all conditions. Data are representative of three independent activation assays; activation levels were normalized to siCon = 1.0 in each assay and expressed as fold activation.
FIGURE 4.
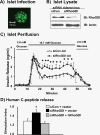
Depletion of RhoGDI from isolated mouse islets augments second-phase insulin secretion. Isolated pancreatic mouse islets were transduced with siRhoGDI adenovirus as described above (multiplicity of infection = 100) and were subjected to confocal fluorescence microscopy using a Zeiss 510 confocal microscope using single-channel scanning with a ×100 objective (bar scale, 100 μm) (A); solubilized in SDS sample buffer and proteins resolved on 12% SDS-PAGE for immunoblotting (IB) (data representative of three independent experiments) (B); and subjected to perifusion analysis at 1-min intervals with 2.8 or 16.7 m
m
glucose as depicted (data are the average ± S.E. of three independently isolated sets of islets; *, p < 0.05 compared with siCon) (C). Human proinsulin DNA plasmid, control, or RhoGDI siRNA oligonucleotides (siCon and siRhoGDI, respectively) were co-transfected into MIN6 cells with human RhoGDI plasmid or empty vector as described under “Experimental Procedures” (D). After 48 h of incubation, cells were preincubated in MKRBB followed by stimulation with 20 m
m
glucose. Human C-peptide secreted into the buffer from the transfected cells was measured by RIA. Data represent the average ± S.E. of at least four independent experiments; *, p < 0.05 versus siCon + vector.
FIGURE 5.
Glucose rapidly induces Cdc42-RhoGDI dissociation in MIN6 beta cells. A, MIN6 cells were incubated in MKRBB for 2 h and left unstimulated or stimulated with 20 m
m
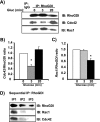
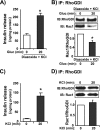
glucose (Gluc) for 3 or 20 min. Detergent cell lysates were prepared for IP with anti-RhoGDI antibody or control IgG. Immunoprecipitates were subjected to 12% SDS-PAGE for immunoblotting (IB) with antibodies indicated. Data are representative of three independent experiments. B, ratio of Cdc42/RhoGDI binding; C, ratio of Rac1/RhoGDI binding under basal conditions was set equal to 1 for normalization of glucose-stimulated ratio in each of three independent experiments. *, p < 0.05 compared with basal. D, cytosolic fractions prepared from MIN6 cells were subjected to three sequential immunoprecipitation reactions (IP1–3) with anti-RhoGDI antibody. Immunoprecipitated proteins were resolved on 12% SDS-PAGE for immunoblotting with the antibodies indicated. Data are representative of three independent experiments.
FIGURE 6.
Pervanadate treatment ablates Cdc42-RhoGDI association in MIN6 beta cells. MIN6 beta cells were incubated in MKRBB for 2 h prior to incubation with either vehicle or freshly made 0.5 m
m
pervanadate (pV) for 5 min prior to preparation of cleared detergent cell lysates. Lysates were immunoblotted (IB) for the presence of p-ERK and total ERK to validate action of pV treatment (A) and for immunoprecipitation with anti-RhoGDI antibody (B). Immunoprecipitated proteins were separated by 12% SDS-PAGE, transferred to PVDF membrane, and immunoblotted with the antibody indicated. Data are representative of three independent experiments.
FIGURE 7.
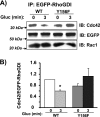
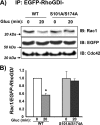
Tyrosine phosphorylation of RhoGDI at Tyr-156 is required for its dissociation from Cdc42 upon glucose stimulation. A, pEGFP-RhoGDI-WT and pEGFP-RhoGDI-Y156F plasmids were transiently expressed in MIN6 cells, after which cells were preincubated in MKRBB followed by glucose (Gluc) stimulation for 3 min. Detergent cell lysates were prepared for immunoprecipitation with anti-EGFP antibody. Immunoprecipitates were resolved on 12% SDS-PAGE for immunoblotting (IB) with the antibodies indicated. Data are representative of three independent experiments. B, ratio of Cdc42 binding to EGFP-RhoGDI proteins under basal conditions was set equal to 1 for normalization of glucose-stimulated ratio in each of three independent experiments; *, p < 0.05 versus basal.
FIGURE 8.
Glucose induces sequential phosphorylation of RhoGDI. MIN6 cells were incubated in MKRBB for 2 h and left unstimulated or stimulated with 20 m
m
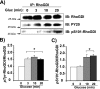
glucose (Gluc) for 3, 10, or 20 min (A). Detergent cell lysates were prepared for IP with anti-RhoGDI antibody or control IgG. Immunoprecipitates were subjected to 12% SDS-PAGE for immunoblotting (IB) with the antibodies indicated. Quantitation of glucose-stimulated phosphorylation of RhoGDI is shown for tyrosine phosphorylation (B) and Ser-101 phosphorylation (C) with respect to total RhoGDI protein. *, p < 0.05 compared with basal set equal to 1 for each of three experiments.
FIGURE 9.
Serine phosphorylation of RhoGDI (Ser-101/Ser-174) is required for its dissociation from Rac1 upon glucose stimulation. A, pEGFP-RhoGDI-WT and pEGFP-RhoGDI-S101A/S174A plasmids were transiently expressed in MIN6 cells, after which cells were preincubated in MKRBB followed by glucose (Gluc) stimulation for 20 min. Detergent cell lysates were prepared for IP with anti-EGFP antibody. Immunoprecipitates were resolved on 12% SDS-PAGE for immunoblotting (IB) with the antibodies indicated. Data are representative of three independent experiments. B, ratio of Rac1 binding to EGFP-RhoGDI proteins under basal conditions was set equal to 1 for normalization of glucose-stimulated ratio in each of three independent experiments. *, p < 0.05 versus basal.
FIGURE 10.
RhoGDI-Rac1 complex dissociation is specific to the glucose-induced amplification phase of insulin release. A, MIN6 beta cells were incubated in glucose-free MKRBB for 2 h, followed by 5 min of incubation with 250 μ
m
diazoxide, addition of 40 m
m
KCl, and then stimulated with 20 m
m d
-glucose (Gluc) for 20 min, and buffer was collected for insulin RIA analysis. B, cells were harvested for anti-RhoGDI immunoprecipitation analysis. Immunoprecipitates were resolved on 12% SDS-PAGE for immunoblotting (IB) with the antibodies indicated. Ratio of Rac1 co-immunoprecipitation with RhoGDI protein under basal conditions was set equal to 1 for normalization of glucose-stimulated ratio in each of three independent experiments; *, p < 0.05 versus basal. C, MIN6 cells were incubated in glucose-free MKRBB for 2 h and subsequently stimulated with 40 m
m
KCl for 20 min. Insulin release (C) and Rac1-RhoGDI ratio (D) were determined as described above. Data represent the average ± S.E. of three independent experiments; *, p < 0.05 versus basal.
FIGURE 11.
Overexpression of RhoGDI specifically inhibited glucose-induced KATP channel-independent insulin secretion. MIN6 cells at 50–60% confluence were co-transfected with 1 μg of pEGFP-vector, pEGFP-RhoGDI-WT, or triple mutant (Y156F/S101A/S174A) plus 1 μg of human proinsulin cDNA. After 48 h of incubation, cells were incubated in glucose-free MKRBB for 2 h and subsequently subjected to diazoxide (250 μ
m
) for 5 min and then stimulated with 20 m
m d
-glucose in the presence of 40 m
m
KCl for 1 h (A) or stimulated with 40 m
m
KCl alone (B). MKRBB was collected for subsequent human C-peptide quantitation by RIA, and each sample was normalized for protein content. Data in each of three independent experiments were normalized to EGFP = 100% and represent the average ± S.E.; *, p < 0.05 versus EGFP; #, p < 0.05 versus WT.
Similar articles
- Cool-1/βPIX functions as a guanine nucleotide exchange factor in the cycling of Cdc42 to regulate insulin secretion.
Kepner EM, Yoder SM, Oh E, Kalwat MA, Wang Z, Quilliam LA, Thurmond DC. Kepner EM, et al. Am J Physiol Endocrinol Metab. 2011 Dec;301(6):E1072-80. doi: 10.1152/ajpendo.00312.2011. Epub 2011 Aug 9. Am J Physiol Endocrinol Metab. 2011. PMID: 21828338 Free PMC article. - Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase.
DerMardirossian C, Schnelzer A, Bokoch GM. DerMardirossian C, et al. Mol Cell. 2004 Jul 2;15(1):117-27. doi: 10.1016/j.molcel.2004.05.019. Mol Cell. 2004. PMID: 15225553 - Disruption of RhoGDI and RhoA regulation by a Rac1 specificity switch mutant.
Wong KW, Mohammadi S, Isberg RR. Wong KW, et al. J Biol Chem. 2006 Dec 29;281(52):40379-88. doi: 10.1074/jbc.M605387200. Epub 2006 Oct 29. J Biol Chem. 2006. PMID: 17074770 - Emerging Roles of Small GTPases in Islet β-Cell Function.
Veluthakal R, Thurmond DC. Veluthakal R, et al. Cells. 2021 Jun 15;10(6):1503. doi: 10.3390/cells10061503. Cells. 2021. PMID: 34203728 Free PMC article. Review. - Small G proteins in islet beta-cell function.
Kowluru A. Kowluru A. Endocr Rev. 2010 Feb;31(1):52-78. doi: 10.1210/er.2009-0022. Epub 2009 Nov 4. Endocr Rev. 2010. PMID: 19890090 Free PMC article. Review.
Cited by
- DOC2b Enhances β-Cell Function via a Novel Tyrosine Phosphorylation-Dependent Mechanism.
Chatterjee Bhowmick D, Aslamy A, Bhattacharya S, Oh E, Ahn M, Thurmond DC. Chatterjee Bhowmick D, et al. Diabetes. 2022 Jun 1;71(6):1246-1260. doi: 10.2337/db21-0681. Diabetes. 2022. PMID: 35377441 Free PMC article. - Mechanisms of the amplifying pathway of insulin secretion in the β cell.
Kalwat MA, Cobb MH. Kalwat MA, et al. Pharmacol Ther. 2017 Nov;179:17-30. doi: 10.1016/j.pharmthera.2017.05.003. Epub 2017 May 18. Pharmacol Ther. 2017. PMID: 28527919 Free PMC article. Review. - Mining for Candidate Genes Related to Pancreatic Cancer Using Protein-Protein Interactions and a Shortest Path Approach.
Yuan F, Zhang YH, Wan S, Wang S, Kong XY. Yuan F, et al. Biomed Res Int. 2015;2015:623121. doi: 10.1155/2015/623121. Epub 2015 Nov 3. Biomed Res Int. 2015. PMID: 26613085 Free PMC article. - Computational analysis of Rho GTPase cycling.
Falkenberg CV, Loew LM. Falkenberg CV, et al. PLoS Comput Biol. 2013;9(1):e1002831. doi: 10.1371/journal.pcbi.1002831. Epub 2013 Jan 10. PLoS Comput Biol. 2013. PMID: 23326220 Free PMC article. - Dyslipidaemia of diabetes and the intestine.
Tomkin GH, Owens D. Tomkin GH, et al. World J Diabetes. 2015 Jul 10;6(7):970-7. doi: 10.4239/wjd.v6.i7.970. World J Diabetes. 2015. PMID: 26185604 Free PMC article. Review.
References
- Rhodes C. J. (2000) in Diabetes Mellitus: A Fundamental and Clinical Text (LeRoith T., Olefsky J. M. eds) pp. 20–38, Lippincott Williams & Wilkins, Philadelphia
- Cook D. L., Hales C. N. (1984) Nature 311, 271–273 - PubMed
- Meglasson M. D., Matschinsky F. M. (1986) Diabetes Metab. Rev. 2, 163–214 - PubMed
- Satin L. S., Cook D. L. (1985) Pflugers Arch. 404, 385–387 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous