Integrins in cancer: biological implications and therapeutic opportunities - PubMed (original) (raw)
Review
Integrins in cancer: biological implications and therapeutic opportunities
Jay S Desgrosellier et al. Nat Rev Cancer. 2010 Jan.
Abstract
The integrin family of cell adhesion receptors regulates a diverse array of cellular functions crucial to the initiation, progression and metastasis of solid tumours. The importance of integrins in several cell types that affect tumour progression has made them an appealing target for cancer therapy. Integrin antagonists, including the alphavbeta3 and alphavbeta5 inhibitor cilengitide, have shown encouraging activity in Phase II clinical trials and cilengitide is currently being tested in a Phase III trial in patients with glioblastoma. These exciting clinical developments emphasize the need to identify how integrin antagonists influence the tumour and its microenvironment.
Figures
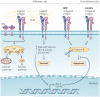
Figure 1. Integrin-mediated survival versus apoptotic pathways
Integrins can paradoxically initiate pro-survival as well as pro-apoptotic signals. Which pathway is more active depends on the ligation status of the surface integrins expressed by a given cell. In a cell in which most of the integrins are ligated, a pro-survival pathway is initiated through increased nuclear factor-κB (NF-κB) or PI3K–AKT activity, decreased p53 activation and increased expression of the pro-survival molecules BCL-2 and FLIP (also known as CFLAR). Cooperative signalling between growth factor receptors and integrins also differentially activates Raf leading to distinct mechanisms of cell survival. Signalling through integrin αvβ3 and the fibroblast growth factor receptor promotes phosphorylation of Ser338 and Ser339 of Raf, protecting cells from the intrinsic pathway of apoptosis, and integrin αvβ5 and vascular endothelial growth factor receptor 2 phosphorylate Tyr340 and Tyr341 of Raf, preventing apoptosis through the extrinsic pathway. In adherent cells in which many of the integrins are unligated, the unligated integrins initiate cleavage of caspase 8, triggering apoptosis through integrin-mediated death (IMD). On complete loss of adhesion, cell death is initiated through a process termed anoikis. Apoptosis induced by anoikis may proceed through either the intrinsic or extrinsic pathways. ECM, extracellular matrix; RTK, receptor tyrosine kinase.
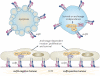
Figure 2. An integrin αvβ3–SRC oncogenic unit promotes anchorage independence
In tumour cells, both β1 integrins (that is, αxβ1) and integrin αvβ3 induce adhesion-dependent activation of focal adhesion kinase (FAK) and SRC, in addition to phosphorylation of the adaptor protein p130 CRK-associated substrate (p130CAS). These signalling events result in invasion, proliferation and survival of tumour cells bound to the extracellular matrix (ECM). In suspended tumour cells unligated integrin αvβ3 signals directly through SRC and p130CAS to increase cell survival independently of FAK. This effect occurs in tumour cells that are already resistant to integrin-mediated death.
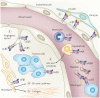
Figure 3. Integrins in the host response to cancer
Integrins expressed in many tumour-associated cell types have crucial roles in increasing tumour progression and metastasis. In endothelial cells, integrins regulate the migration, proliferation and survival necessary for angiogenesis (step 1). The interaction between pericytes and endothelial cells is crucial for the stabilization of newly formed vessels during angiogenesis. Binding of integrin α4β1 on endothelial cells to vascular cell adhesion molecule 1 (VCAM1) on pericytes plays an important part in pericyte recruitment to the neovasculature (step 2). Myeloid cells and monocytes in primary tumours contribute to disease progression by secreting cytokines and growth factors (GFs) that initiate angiogenesis and tumour cell migration (step 3). Several studies have shown that integrins have an essential role in the homing of myeloid cells and monocytes to tumours. Fibroblast infiltration into the primary tumour, known as desmoplasia, also contributes to tumour progression through increased growth factor secretion (step 4). In addition, the invading fibroblasts deposit large amounts of collagen that might result in resistance to therapy in some tumours (step 5). A recent study showed that integrins, such as α11β1, are crucial regulators of growth factor secretion by these fibroblasts. Platelet expression of αIIbβ3 may be important for interacting with tumour cells through a fibrinogen bridge, possibly aiding in metastatic dissemination (step 6).
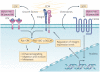
Figure 4. Integrin–growth factor and integrin–cytokine receptor crosstalk
Cooperation between integrin and growth factor signalling or integrin and cytokine signalling is crucial to tumour progression. Several crosstalk mechanisms have so far been elucidated. Integrin ligation may lead directly to the increased secretion of growth factors and/or cytokines, which can then bind to their receptors in an autocrine or paracrine manner to further induce signalling. In addition, signalling induced by either integrin ligation or growth factor binding may activate common downstream pathways resulting in enhanced signalling overall compared with the activation of either receptor alone. This signalling seems to most commonly converge on kinases such as Src family kinases (SFKs), scaffolding proteins such as p130 CRK-associated substrate (p130CAS), and GTPases, such as the Ras family. Alternatively, both chemokine and growth factor signalling may regulate integrin function by directly controlling integrin expression levels. ECM, extracellular matrix; EGF, epidermal growth factor; FAK, focal adhesion kinase; SDF1, stromal cell-derived factor 1; RTK, receptor tyrosine kinase.
Similar articles
- Small molecule integrin antagonists in cancer therapy.
Paolillo M, Russo MA, Serra M, Colombo L, Schinelli S. Paolillo M, et al. Mini Rev Med Chem. 2009 Oct;9(12):1439-46. doi: 10.2174/138955709789957404. Mini Rev Med Chem. 2009. PMID: 19929817 Review. - Targeting integrins in malignant glioma.
Tabatabai G, Weller M, Nabors B, Picard M, Reardon D, Mikkelsen T, Ruegg C, Stupp R. Tabatabai G, et al. Target Oncol. 2010 Sep;5(3):175-81. doi: 10.1007/s11523-010-0156-3. Epub 2010 Sep 4. Target Oncol. 2010. PMID: 20820929 Review. - Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results.
Scaringi C, Minniti G, Caporello P, Enrici RM. Scaringi C, et al. Anticancer Res. 2012 Oct;32(10):4213-23. Anticancer Res. 2012. PMID: 23060541 Review. - Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme.
Reardon DA, Nabors LB, Stupp R, Mikkelsen T. Reardon DA, et al. Expert Opin Investig Drugs. 2008 Aug;17(8):1225-35. doi: 10.1517/13543784.17.8.1225. Expert Opin Investig Drugs. 2008. PMID: 18616418 Free PMC article. Review. - The role of integrins in glioma biology and anti-glioma therapies.
Tabatabai G, Tonn JC, Stupp R, Weller M. Tabatabai G, et al. Curr Pharm Des. 2011;17(23):2402-10. doi: 10.2174/138161211797249189. Curr Pharm Des. 2011. PMID: 21827415 Review.
Cited by
- Combination strategies with PARP inhibitors in BRCA-mutated triple-negative breast cancer: overcoming resistance mechanisms.
Jain A, Barge A, Parris CN. Jain A, et al. Oncogene. 2024 Nov 21. doi: 10.1038/s41388-024-03227-6. Online ahead of print. Oncogene. 2024. PMID: 39572842 Review. - Serum small extracellular vesicles-derived BST2 as a biomarker for papillary thyroid microcarcinoma promotes lymph node metastasis.
Cao Z, Wang Y, Wu J, Tang X, Qian Z, Zhang Z, Liu R, Liu P, Li Z, Xu X, Liu Z. Cao Z, et al. Cancer Gene Ther. 2024 Nov 18. doi: 10.1038/s41417-024-00854-9. Online ahead of print. Cancer Gene Ther. 2024. PMID: 39558134 - Exosomal integrins in tumor progression, treatment and clinical prediction (Review).
Shen YQ, Sun L, Wang SM, Zheng XY, Xu R. Shen YQ, et al. Int J Oncol. 2024 Dec;65(6):118. doi: 10.3892/ijo.2024.5706. Epub 2024 Nov 14. Int J Oncol. 2024. PMID: 39540373 Free PMC article. Review. - Colorectal carcinoma progression is not influenced by the pseudokinase PEAK1.
Zuidema A, Atherton P, van der Poel S, Kreft M, Song JY, Bierbooms M, Verhoeven S, Papagianni C, Kroese L, Ali RB, Huijbers I, Carvalho B, Sonnenberg A. Zuidema A, et al. Sci Rep. 2024 Nov 12;14(1):27663. doi: 10.1038/s41598-024-78776-7. Sci Rep. 2024. PMID: 39532961 Free PMC article. - Repurposing the antipsychotic drug penfluridol for cancer treatment (Review).
Ali Ibrahim Mze A, Abdul Rahman A. Ali Ibrahim Mze A, et al. Oncol Rep. 2024 Dec;52(6):174. doi: 10.3892/or.2024.8833. Epub 2024 Nov 8. Oncol Rep. 2024. PMID: 39513619 Free PMC article. Review.
References
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. - PubMed
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nature Rev. Mol. Cell Biol. 2004;5:816–826. - PubMed
- Han S, Khuri FR, Roman J. Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res. 2006;66:315–323. - PubMed
- Vellon L, Menendez JA, Lupu R. αVβ3 integrin regulates heregulin (HRG)-induced cell proliferation and survival in breast cancer. Oncogene. 2005;24:3759–3773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA121938/CA/NCI NIH HHS/United States
- R37 CA050286/CA/NCI NIH HHS/United States
- R01 CA045726/CA/NCI NIH HHS/United States
- CA45726/CA/NCI NIH HHS/United States
- R01 CA050286/CA/NCI NIH HHS/United States
- CA50286/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources