Senescence in tumours: evidence from mice and humans - PubMed (original) (raw)
Review
Senescence in tumours: evidence from mice and humans
Manuel Collado et al. Nat Rev Cancer. 2010 Jan.
Abstract
The importance of cellular senescence, which is a stress response that stably blocks proliferation, is increasingly being recognized. Senescence is prevalent in pre-malignant tumours, and progression to malignancy requires evading senescence. Malignant tumours, however, may still undergo senescence owing to interventions that restore tumour suppressor function or inactivate oncogenes. Senescent tumour cells can be cleared by immune cells, which may result in efficient tumour regression. Standard chemotherapy also has the potential to induce senescence, which may partly underlie its therapeutic activity. Although these concepts are well supported in mouse models, translating them to clinical oncology remains a challenge.
Figures
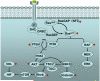
Figure 1
Signalling pathways and oncogenes (marked by a red asterisk) whose activation leads to senescence induction in vivo.
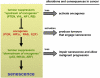
Figure 2
Tumour suppressors can be grouped into two categories depending on their effect on senescence. Some tumour suppressors (at the top of the cascade, i.e. “upstream of oncogenes”) prevent excessive oncogenic signalling and their deletion in normal cells triggers senescence. Other tumour suppressors (“downstream of oncogenes”) sense excessive oncogenic signalling and induce senescence, and their absence in tumour cells allows progression to malignancy.
Similar articles
- Cellular senescence in the development and treatment of cancer.
Saretzki G. Saretzki G. Curr Pharm Des. 2010 Jan;16(1):79-100. doi: 10.2174/138161210789941874. Curr Pharm Des. 2010. PMID: 20214620 Review. - Distinct phenotypes and 'bystander' effects of senescent tumour cells induced by docetaxel or immunomodulatory cytokines.
Sapega O, Mikyšková R, Bieblová J, Mrázková B, Hodný Z, Reiniš M. Sapega O, et al. Int J Oncol. 2018 Nov;53(5):1997-2009. doi: 10.3892/ijo.2018.4553. Epub 2018 Sep 5. Int J Oncol. 2018. PMID: 30226595 Free PMC article. - Senescence-associated secretory factors induced by cisplatin in melanoma cells promote non-senescent melanoma cell growth through activation of the ERK1/2-RSK1 pathway.
Sun X, Shi B, Zheng H, Min L, Yang J, Li X, Liao X, Huang W, Zhang M, Xu S, Zhu Z, Cui H, Liu X. Sun X, et al. Cell Death Dis. 2018 Feb 15;9(3):260. doi: 10.1038/s41419-018-0303-9. Cell Death Dis. 2018. PMID: 29449532 Free PMC article. - The power and the promise of oncogene-induced senescence markers.
Collado M, Serrano M. Collado M, et al. Nat Rev Cancer. 2006 Jun;6(6):472-6. doi: 10.1038/nrc1884. Nat Rev Cancer. 2006. PMID: 16723993 - [Senescence and cellular immortality].
Trentesaux C, Riou JF. Trentesaux C, et al. Bull Cancer. 2010 Nov;97(11):1275-83. doi: 10.1684/bdc.2010.1205. Bull Cancer. 2010. PMID: 21051314 Review. French.
Cited by
- Therapeutic Effect of Icaritin on Cerebral Ischemia-Reperfusion-Induced Senescence and Apoptosis in an Acute Ischemic Stroke Mouse Model.
Wu CT, Yang TH, Chen MC, Guan SS, Chen CM, Liu SH. Wu CT, et al. Molecules. 2022 Sep 7;27(18):5783. doi: 10.3390/molecules27185783. Molecules. 2022. PMID: 36144517 Free PMC article. - Inactivation of heat shock factor Hsf4 induces cellular senescence and suppresses tumorigenesis in vivo.
Jin X, Eroglu B, Cho W, Yamaguchi Y, Moskophidis D, Mivechi NF. Jin X, et al. Mol Cancer Res. 2012 Apr;10(4):523-34. doi: 10.1158/1541-7786.MCR-11-0530. Epub 2012 Feb 21. Mol Cancer Res. 2012. PMID: 22355043 Free PMC article. - Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation.
Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P. Barascu A, et al. EMBO J. 2012 Mar 7;31(5):1080-94. doi: 10.1038/emboj.2011.492. Epub 2012 Jan 13. EMBO J. 2012. PMID: 22246186 Free PMC article. - Conditional expression of oncogenic C-RAF in mouse pulmonary epithelial cells reveals differential tumorigenesis and induction of autophagy leading to tumor regression.
Ceteci F, Xu J, Ceteci S, Zanucco E, Thakur C, Rapp UR. Ceteci F, et al. Neoplasia. 2011 Nov;13(11):1005-18. doi: 10.1593/neo.11652. Neoplasia. 2011. PMID: 22131876 Free PMC article. - Therapeutic Senolysis of Axitinib-Induced Senescent Human Lung Cancer Cells.
Kotani H, Han W, Iida Y, Tanino R, Katakawa K, Okimoto T, Tsubata Y, Isobe T, Harada M. Kotani H, et al. Cancers (Basel). 2024 Aug 7;16(16):2782. doi: 10.3390/cancers16162782. Cancers (Basel). 2024. PMID: 39199555 Free PMC article.
References
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. - PubMed
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. - PubMed
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. Original description of oncogene-induced senescence in in vitro cultured primary human and mouse cells after overexpression of oncogenic HRAS. Prompted the concept of cellular senescence as a tumour suppressor mechanism.
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. - PubMed
- Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases