Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice - PubMed (original) (raw)
Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice
Bridgette D Semple et al. J Cereb Blood Flow Metab. 2010 Apr.
Abstract
Cerebral inflammation involves molecular cascades contributing to progressive damage after traumatic brain injury (TBI). The chemokine CC ligand-2 (CCL2) (formerly monocyte chemoattractant protein-1, MCP-1) is implicated in macrophage recruitment into damaged parenchyma after TBI. This study analyzed the presence of CCL2 in human TBI, and further investigated the role of CCL2 in physiological and cellular mechanisms of secondary brain damage after TBI. Sustained elevation of CCL2 was detected in the cerebrospinal fluid (CSF) of severe TBI patients for 10 days after trauma, and in cortical homogenates of C57Bl/6 mice, peaking at 4 to 12 h after closed head injury (CHI). Neurological outcome, lesion volume, macrophage/microglia infiltration, astrogliosis, and the cerebral cytokine network were thus examined in CCL2-deficient (-/-) mice subjected to CHI. We found that CCL2-/- mice showed altered production of multiple cytokines acutely (2 to 24 h); however, this did not affect lesion size or cell death within the first week after CHI. In contrast, by 2 and 4 weeks, a delayed reduction in lesion volume, macrophage accumulation, and astrogliosis were observed in the injured cortex and ipsilateral thalamus of CCL2-/- mice, corresponding to improved functional recovery as compared with wild-type mice after CHI. Our findings confirm the significant role of CCL2 in mediating post-traumatic secondary brain damage.
Figures
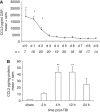
Figure 1
The time profiles of CCL2 production after TBI in humans and mice. CSF from severe TBI patients (_n_=21) was collected by drainage of the intraventricular catheter from day 0 (day of admission) to day 9, and CCL2 levels were measured by ELISA (A). A significant increase in CCL2 was evident on day 0, which remained elevated at day 1 and 2, as compared with subsequent times (*P<0.001). CCL2 levels were consistently higher in trauma patients as compared with those in control patients (dotted line; 717.01±71.5 pg/mL; P<0.001). CCL2 was also measured in the homogenates of lesioned cortex from wt mice (_n_=5) up to 24 h after CHI (B). CCL2 levels were significantly enhanced at 4 and 12 h after CHI as compared with those in sham-operated mice (**P<0.01). In both patients and mice, CCL2 levels peaked within the first 24 h. Data are expressed as mean+s.e.m.
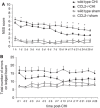
Figure 2
CCL2−/− mice have improved functional recovery after CHI. The NSS reflects motor function, alertness, and behavior, with a score of 10 indicating the most severe dysfunction (A). CCL2−/− mice (_n_=8) have similar scores at 1 h after injury as compared with wt mice (_P_=0.347, _n_=9), and show a comparable rate of recovery up to 6 days after CHI. However, CCL2−/− mice showed improved neurological recovery, with significantly lower NSS scores compared with wt mice at 1, 7, 10, 14, 17, 21 and 24 days after CHI (*P<0.05, Tukey's post hoc). No differences were observed between sham-operated mice of each strain throughout the study period (_P_=0.863, _n_=4 to 5). The ledged beam test was also used to assess motor deficit of limbs contralateral to the lesioned cortex (B). The number of errors tended to be lower overall in CCL2−/− mice as compared with that in wt mice after CHI (_P_=0.115). Data are expressed as mean+s.e.m.
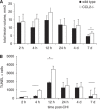
Figure 3
Lesion volume and cell death in wt and CCL2−/− mice over the acute time course. No differences were detected in total lesion volume between wt and CCL2−/− brains, as assessed using H&E-stained sections over 7 days after CHI (A; _P_=0.275, two-way ANOVA; _n_=6 to 7). By 7 days, lesion volume had decreased overall regardless of strain as compared with the lesion volume in the first 12 h after injury (*P<0.05; 12 h versus 7 days). Cell death was assessed by TUNEL staining using sections adjacent to H&E (B). Note the typical time course of cell death after TBI, with minimal positive cells at 2 and 4 h, followed by a dramatic peak of cell death at 12 h (P<0.05; 12 versus 2 h, 4 h, and 7 days). Consistent with lesion volume measurements, quantification of TUNEL labeling showed similar numbers of dead/dying cells in wt and CCL2−/− brains (_P_=0.059, two-way ANOVA).
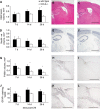
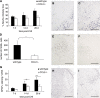
Figure 4
Immunohistochemical analysis of the injured hemisphere in wt and CCL2−/− mice over the delayed time course. Total lesion volumes in wt and CCL2−/− mice was assessed using H&E-stained sections (A; _n_=6 to 7, from 7 to 28 days). Significantly reduced lesion volumes were observed in CCL2−/− mice overall (_P_=0.047, two-way ANOVA), particularly at 28 days (*P<0.05, Tukey's post hoc). Representative wt and CCL2−/− sections (B, C) show the evident difference in lesion size at 28 days. The loss of NeuN-positive neurons (D) coincided with changes in lesion volume, with a trend toward reduced neuronal loss in CCL2−/− mice (F) at 28 days as compared with wt mice (E; _P_=0.088). Quantification of macrophages and reactive microglia was expressed as volume of F4/80-positive cell spread in the injured cortex (G). Despite similar volumes in both strains up to 7 days after CHI, by 14 and 28 days the volume of F4/80-positive cells was significantly reduced in CCL2−/− mice (_P_=0.007 overall, two-way ANOVA; *P<0.05, Tukey's post hoc), as shown in representative sections of wt (H) and CCL2−/− mice (I). Density of GFAP-positive astrocytes was calculated as the percentage of the cortical ROI (J). Across the time course, GFAP immunoreactivity in CCL2−/− mice was reduced by approximately 30% as compared with that in wt mice (_P_=0.026, two-way ANOVA). This difference is evident in representative sections from wt (K) and CCL2−/− mice at 28 days (L). Data are expressed as mean+s.e.m. Scale bar=1,000 _μ_m in panels B, C, E, and F; 500 _μ_m in panels H, I, K, and L. Color figure provided as Supplementary Information.
Figure 5
Neuronal loss and glial activation in the ipsilateral thalamus of wt and CCL2−/− mice. Neuronal loss in the ipsilateral dorsal thalamus was determined as % NeuN-positive staining of the uninjured contralateral thalamus (A; _n_=6 to 7). Overall, wt mice had a greater loss of NeuN staining as compared with CCL2−/− mice (_P_=0.032, two-way ANOVA), as shown in representative sections (B, C; wt and CCL2−/− mice at 28 days, respectively). The number of F4/80-positive cells was counted in the thalamus at 28 days (D). While abundant reactive microglia were observed in wt mice (E), CCL2−/− mice (F) had significantly fewer F4/80-positive cells (*P<0.05, _t_-test; _n_=7). Astrocyte activation in the thalamus was expressed as the percentage of the ROI stained with GFAP (G). GFAP-staining intensity increased over time in both strains (**P<0.01, ***P<0.001, versus 7 days; two-way ANOVA effect of time). However, CCL2−/− mice had overall less astrocyte reactivity as compared with that in wt mice (_P_=0.026). Representative images of the GFAP-stained ipsilateral thalamus are shown for wt (H) and CCL2−/− (I) mice at 28 days. Data are expressed as mean+s.e.m. Scale bar=200 _μ_m. Color figure provided as Supplementary Information.
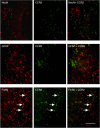
Figure 6
Localization of CCR2 receptor on F4/80-positive amoeboid macrophages. Double-labeling immunofluorescence of CCR2, in combination with NeuN, GFAP, or F4/80 illustrates the cellular localization of CCR2 at 4 days after CHI in wt mice. In the pericontusional cortex adjacent to the lesion core, no colocalization of NeuN or GFAP was detected with CCR2 staining (upper and middle panels). In contrast, CCR2 expression was evident within a subset of F4/80-positive amoeboid macrophages accumulating in the lesion (lower panels; arrows). Scale bar=100 _μ_m.
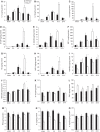
Figure 7
Inflammatory cytokine/chemokine expression in brain homogenates after CHI. The levels of 15 cytokines were detected in the homogenates of lesioned cortex over 24 h after CHI in wt and CCL2−/− mice (n_=5). Production of the cytokines/chemokines IL-1_α, IL-1_β_, IL-6, granulocyte colony-stimulating factor, IL-12(p40), MIP-1_α_, KC, and MIP-2 (A–H) was maximal and delayed to 12 h in CCL2−/− mice (white bars) as compared with that in wt mice (black bars), in which they peaked by 4 h (*P<0.05, wt versus CCL2−/− at 12 h, Tukey's post hoc). The chemokine RANTES (I) was reduced in CCL2−/− mice as compared with wt mice, particularly in sham-operated mice and at 4 h after CHI (*P<0.05). Cytokines GM-CSF and IL-10 (J, K) were significantly elevated in CCL2−/− (white bars) mice as compared with wt mice (black bars) at 12 h post CHI (*P<0.05). While TNF_α_ did not appear to be induced by CHI (L), overall the level of this cytokine was significantly higher in CCL2−/− mice, even in sham-operated controls (P<0.001, two-way ANOVA). In contrast, the production of interferon-γ, IL-2, and IL-12(p70) (M, N, and O) was reduced in CCL2−/− mice as compared with that in wt controls over the time course (_P_=0.017, 0.001, and 0.033, respectively). Data are expressed as pg/mg protein (mean+s.e.m.).
Similar articles
- The CCL2/CCL7/CCL12/CCR2 pathway is substantially and persistently upregulated in mice after traumatic brain injury, and CCL2 modulates the complement system in microglia.
Popiolek-Barczyk K, Ciechanowska A, Ciapała K, Pawlik K, Oggioni M, Mercurio D, De Simoni MG, Mika J. Popiolek-Barczyk K, et al. Mol Cell Probes. 2020 Dec;54:101671. doi: 10.1016/j.mcp.2020.101671. Epub 2020 Nov 4. Mol Cell Probes. 2020. PMID: 33160071 - The scavenging chemokine receptor ACKR2 has a significant impact on acute mortality rate and early lesion development after traumatic brain injury.
Woodcock TM, Frugier T, Nguyen TT, Semple BD, Bye N, Massara M, Savino B, Besio R, Sobacchi C, Locati M, Morganti-Kossmann MC. Woodcock TM, et al. PLoS One. 2017 Nov 27;12(11):e0188305. doi: 10.1371/journal.pone.0188305. eCollection 2017. PLoS One. 2017. PMID: 29176798 Free PMC article. - Ccr2 Gene Ablation Does Not Influence Seizure Susceptibility, Tissue Damage, or Cellular Inflammation after Murine Pediatric Traumatic Brain Injury.
Sharma R, Chu E, Dill LK, Shad A, Zamani A, O'Brien TJ, Casillas-Espinosa PM, Shultz SR, Semple BD. Sharma R, et al. J Neurotrauma. 2023 Feb;40(3-4):365-382. doi: 10.1089/neu.2022.0033. Epub 2022 Nov 8. J Neurotrauma. 2023. PMID: 36070444 - The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials.
Geng H, Chen L, Tang J, Chen Y, Wang L. Geng H, et al. Int J Mol Sci. 2022 Mar 23;23(7):3485. doi: 10.3390/ijms23073485. Int J Mol Sci. 2022. PMID: 35408846 Free PMC article. Review. - [Progress on Hypoxic-ischemic Brain Damage Associated with CCR2 and CCL2].
Luo YJ, Li RB, Ma SY, Lü MY. Luo YJ, et al. Fa Yi Xue Za Zhi. 2016 Feb;32(1):54-7. Fa Yi Xue Za Zhi. 2016. PMID: 27295859 Review. Chinese.
Cited by
- Glial biomarkers in human central nervous system disease.
Garden GA, Campbell BM. Garden GA, et al. Glia. 2016 Oct;64(10):1755-71. doi: 10.1002/glia.22998. Epub 2016 May 26. Glia. 2016. PMID: 27228454 Free PMC article. Review. - The effect of nicotinamide on gene expression in a traumatic brain injury model.
Anderson GD, Peterson TC, Farin FM, Bammler TK, Beyer RP, Kantor ED, Hoane MR. Anderson GD, et al. Front Neurosci. 2013 Feb 26;7:21. doi: 10.3389/fnins.2013.00021. eCollection 2013. Front Neurosci. 2013. PMID: 23550224 Free PMC article. - The role of markers of inflammation in traumatic brain injury.
Woodcock T, Morganti-Kossmann MC. Woodcock T, et al. Front Neurol. 2013 Mar 4;4:18. doi: 10.3389/fneur.2013.00018. eCollection 2013. Front Neurol. 2013. PMID: 23459929 Free PMC article. - Biomarkers in traumatic brain injury.
Sharma R, Laskowitz DT. Sharma R, et al. Curr Neurol Neurosci Rep. 2012 Oct;12(5):560-9. doi: 10.1007/s11910-012-0301-8. Curr Neurol Neurosci Rep. 2012. PMID: 22811071 Review. - Amount of Mononuclear Phagocyte Infiltrate Does Not Predict Area of Experimental Choroidal Neovascularization (CNV).
Will-Orrego A, Qiu Y, Fassbender ES, Shen S, Aranda J, Kotagiri N, Maker M, Liao SM, Jaffee BD, Poor SH. Will-Orrego A, et al. J Ocul Pharmacol Ther. 2018 Sep;34(7):489-499. doi: 10.1089/jop.2017.0131. J Ocul Pharmacol Ther. 2018. PMID: 30188257 Free PMC article.
References
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM. Distribution, cellular localisation and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81:257–269. - PubMed
- Beni-Adani L, Gozes I, Cohen Y, Assaf Y, Steingart RA, Brenneman DE, Eizenberg O, Trembolver V, Shohami E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther. 2001;296:57–63. - PubMed
- Berman JW, Guida MP, Warren J, Amat J, Brosnan CF. Localisation of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol. 1996;156:3017–3023. - PubMed
- Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RS, Kochanek PM. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1717. - PubMed
- Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204:220–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous