Imaging cortical dopamine D1 receptors using [11C]NNC112 and ketanserin blockade of the 5-HT 2A receptors - PubMed (original) (raw)
Imaging cortical dopamine D1 receptors using [11C]NNC112 and ketanserin blockade of the 5-HT 2A receptors
Ana M Catafau et al. J Cereb Blood Flow Metab. 2010 May.
Abstract
[(11)C]NNC112 (8-chloro-7-hydroxy-3-methyl-5-(7-benzofuranyl)-2,3,4,5-tetrahydro-IH-3-benzazepine), a selective positron-emission tomography (PET) ligand for the D(1) receptor (R) over the 5-HT(2A) R in vitro, has shown lower selectivity in vivo, hampering measurement of D(1) R in the cortex. [(11)C]NNC112 PET and intravenous (i.v) ketanserin challenge were used to (1) confirm the previous findings of [(11)C]NNC112 in vivo D(1) R selectivity, and (2) develop a feasible methodology for imaging cortical D(1) R without contamination by 5-HT(2A) R. Seven healthy volunteers underwent [(11)C]NNC112 PET scans at baseline and after a 5-HT(2A) R-blocking dose of ketanserin (0.15 mg/kg, i.v.). Percent BP(ND) change between the post-ketanserin and baseline scans was calculated. Irrespective of the quantification method used, ketanserin pretreatment led to significant decrease of BP(ND) in the cortical (approximately 30%) and limbic regions (approximately 20%) but not in the striatum, which contains a much lower amount of 5-HT(2A) R. Therefore, ketanserin allows D(1) R signal to be detected by [(11)C]NNC112 PET without significant 5-HT(2A) R contamination. These data confirm the presence of a significant 5-HT(2A) R contribution to cortical [(11)C]NNC112 signal, and call for caution in the interpretation of published [(11)C]NNC112 PET findings on cortical D(1) R in humans. In the absence of more selective ligands, [(11)C]NNC112 PET with ketanserin can be used for cortical D(1) R imaging in vivo.
Figures
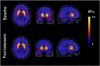
Figure 1
Co-registered MRI: [11C]NNC112 PET _BP_ND parametric images (mean from all subjects, _n_=6), at baseline (top) and after ketanserin 0.15 mg/kg, i.v. (bottom). The decrease of [11C]NNC112 signal in the cortical regions, without modification in the striatum, is appreciated.
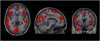
Figure 2
T maps of _BP_ND change between baseline and post-ketanserin conditions. Threshold: P<0.001. A significant _BP_ND decrease was shown in the cortical areas, demonstrating [11C]NNC112 displacement from 5-HT2A R, whereas no significant changes are seen in the striatum (area devoid of 5-HT2A R).
Similar articles
- In vivo binding of the dopamine-1 receptor PET tracers [¹¹C]NNC112 and [¹¹C]SCH23390: a comparison study in individuals with schizophrenia.
Poels EM, Girgis RR, Thompson JL, Slifstein M, Abi-Dargham A. Poels EM, et al. Psychopharmacology (Berl). 2013 Jul;228(1):167-74. doi: 10.1007/s00213-013-3026-8. Epub 2013 Mar 5. Psychopharmacology (Berl). 2013. PMID: 23460265 - Nicotine normalizes increased prefrontal cortical dopamine D1 receptor binding and decreased working memory performance produced by repeated pretreatment with MK-801: a PET study in conscious monkeys.
Tsukada H, Miyasato K, Nishiyama S, Fukumoto D, Kakiuchi T, Domino EF. Tsukada H, et al. Neuropsychopharmacology. 2005 Dec;30(12):2144-53. doi: 10.1038/sj.npp.1300745. Neuropsychopharmacology. 2005. PMID: 15856080 - Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride.
Slifstein M, Suckow RF, Javitch JA, Cooper T, Lieberman J, Abi-Dargham A. Slifstein M, et al. J Cereb Blood Flow Metab. 2011 Jan;31(1):293-304. doi: 10.1038/jcbfm.2010.91. Epub 2010 Jun 23. J Cereb Blood Flow Metab. 2011. PMID: 20571519 Free PMC article. - Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia.
Kosaka J, Takahashi H, Ito H, Takano A, Fujimura Y, Matsumoto R, Nozaki S, Yasuno F, Okubo Y, Kishimoto T, Suhara T. Kosaka J, et al. Life Sci. 2010 May 22;86(21-22):814-8. doi: 10.1016/j.lfs.2010.03.018. Epub 2010 Mar 30. Life Sci. 2010. PMID: 20361984 - Ketanserin and spiperone as templates for novel serotonin 5-HT(2A) antagonists.
Glennon RA, Metwally K, Dukat M, Ismaiel AM, De los Angeles J, Herndon J, Teitler M, Khorana N. Glennon RA, et al. Curr Top Med Chem. 2002 Jun;2(6):539-58. doi: 10.2174/1568026023393787. Curr Top Med Chem. 2002. PMID: 12052193 Review.
Cited by
- Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects.
Pilar-Cuéllar F, Vidal R, Pazos A. Pilar-Cuéllar F, et al. Br J Pharmacol. 2012 Feb;165(4b):1046-57. doi: 10.1111/j.1476-5381.2011.01516.x. Br J Pharmacol. 2012. PMID: 21627639 Free PMC article. - Glutamate and dopamine in schizophrenia: an update for the 21st century.
Howes O, McCutcheon R, Stone J. Howes O, et al. J Psychopharmacol. 2015 Feb;29(2):97-115. doi: 10.1177/0269881114563634. Epub 2015 Jan 13. J Psychopharmacol. 2015. PMID: 25586400 Free PMC article. Review. - The direct basal ganglia pathway is hyperfunctional in focal dystonia.
Simonyan K, Cho H, Hamzehei Sichani A, Rubien-Thomas E, Hallett M. Simonyan K, et al. Brain. 2017 Dec 1;140(12):3179-3190. doi: 10.1093/brain/awx263. Brain. 2017. PMID: 29087445 Free PMC article. - Impact of image-based motion correction on dopamine D3/D2 receptor occupancy-comparison of groupwise and frame-by-frame registration approaches.
Jiao J, Searle GE, Schnabel JA, Gunn RN. Jiao J, et al. EJNMMI Phys. 2015 Dec;2(1):15. doi: 10.1186/s40658-015-0117-0. Epub 2015 Jul 29. EJNMMI Phys. 2015. PMID: 26501816 Free PMC article. - Kinetic Modelling and Test-Retest Reproducibility for the Dopamine D1R Radioligand [11C]SCH23390 in Healthy and Diseased Mice.
Bertoglio D, Verhaeghe J, Miranda A, Wyffels L, Stroobants S, Dominguez C, Munoz-Sanjuan I, Skinbjerg M, Liu L, Staelens S. Bertoglio D, et al. Mol Imaging Biol. 2021 Apr;23(2):208-219. doi: 10.1007/s11307-020-01561-1. Epub 2020 Nov 11. Mol Imaging Biol. 2021. PMID: 33179158 Free PMC article.
References
- Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20:225–243. - PubMed
- Andersen PH. Comparison of the pharmacological characteristics of [3H]raclopride and [3H]SCH 23390 binding to dopamine receptors in vivo in mouse brain. Eur J Pharmacol. 1988;146:113–120. - PubMed
- Andersen PH, Gronvald FC, Hohlweg R, Hansen LB, Guddal E, Braestrup C, Nielsen EB. NNC-112, NNC-687 and NNC-756, new selective and highly potent dopamine D1 receptor antagonists. Eur J Pharmacol. 1992;219:45–52. - PubMed
- Catafau AM, Danus M, Bullich S, Llop J, Perich J, Cunningham VJ, Plaza P, Penengo MM, Eersels JL, Squassante L, Ros D, Barbanoj M. Characterization of the SPECT 5-HT2A receptor ligand 123I-R91150 in healthy volunteers: part 1—pseudoequilibrium interval and quantification methods. J Nucl Med. 2006a;47:919–928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous