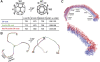Structure of clathrin coat with bound Hsc70 and auxilin: mechanism of Hsc70-facilitated disassembly - PubMed (original) (raw)
Structure of clathrin coat with bound Hsc70 and auxilin: mechanism of Hsc70-facilitated disassembly
Yi Xing et al. EMBO J. 2010.
Abstract
The chaperone Hsc70 drives the clathrin assembly-disassembly cycle forward by stimulating dissociation of a clathrin lattice. A J-domain containing co-chaperone, auxilin, associates with a freshly budded clathrin-coated vesicle, or with an in vitro assembled clathrin coat, and recruits Hsc70 to its specific heavy-chain-binding site. We have determined by electron cryomicroscopy (cryoEM), at about 11 A resolution, the structure of a clathrin coat (in the D6-barrel form) with specifically bound Hsc70 and auxilin. The Hsc70 binds a previously analysed site near the C-terminus of the heavy chain, with a stoichiometry of about one per three-fold vertex. Its binding is accompanied by a distortion of the clathrin lattice, detected by a change in the axial ratio of the D6 barrel. We propose that when Hsc70, recruited to a position close to its target by the auxilin J-domain, splits ATP, it clamps firmly onto its heavy-chain site and locks in place a transient fluctuation. Accumulation of the local strain thus imposed at multiple vertices can then lead to disassembly.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
Components of the clathrin uncoating process. (A) Domain organization of Hsc70 (top), auxilin (middle) and clathrin heavy chain (bottom). Residue numbers for domain or regional boundaries are shown below the bars. (B) A clathrin triskelion (left) and its packing within the lattice of a coat (right). The various regions of the heavy chain are labelled; the ordered, 71-residue α-helical segment of the light chain is also shown. Three symmetry-distinct vertices are colour-coded, yellow, blue (the hub of the blue triskelion) and green. (C) Side view of the triskelion (left), illustrating the pucker at the apex, and a close-up of the hub region, including the helical tripod and the QLMLT sequence near the C-terminus.
Figure 2
Tight, auxilin-specific binding of Hsc70 depends on ATP hydrolysis. (A) SDS–PAGE of resuspended high-speed pellet from preparation of coats, bound with saturating amounts of auxilin (547–910) and incubated with increasing concentrations of Hsc70:ATP (lanes 1–4) or Hsc70:ADP (lanes 5–8). See Materials and methods for details. (B) Hsc70 associated with coats has hydrolysed ATP. TLC analysis showing 32P-labelled nucleotide in the mixture at the time of Hsc70:ATP addition and after separation by centrifugation into supernatant (free Hsc70 with both free and bound nucleotides) and pellet (Hsc70 and nucleotide bound to coats).
Figure 3
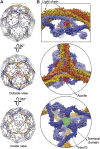
Image reconstruction of an Hsc70 (1–554):auxilin (547–910):clathrin coat. (A) Outside view (left) and cutaway view (right) of the complete coat. Clathrin is in blue, auxilin (547–910) is in red and Hsc70 (1–554) is in green. The boundaries of clathrin and the auxilin fragment are as in Fotin et al (2004b). The boundary of the Hsc70 was determined by comparing the new reconstruction with the previously published reconstruction of the auxilin complex. (B) Detailed views of the density map in specific regions, to illustrate the helical zig-zag and the fit of the heavy-chain model.
Figure 4
Invariance of the proximal–distal contact. (A) The 8 Å resolution map of the D6 coat (Fotin et al, 2004a), with the model of corresponding heavy-chain segments. The view is in a direction tangential to the surface of a coat, with the exterior of the lattice above and the interior below. (B) Corresponding map and model for the Hsc70:auxilin:clathrin complex. (C) Superposition of the two, with the map from the uncomplexed coat in blue (as in A) and the map from the ternary complex in brown (as in B). The two maps were positioned to optimize agreement in the proximal-leg region, and the excellent superposition of the distal-leg maps shows that the interface does not shift when the ligands distort the coat.
Figure 5
Relative positions of auxilin (547–910) and Hsc70 (1–554) in the complex. (A) Overview of the D6 coat, showing in dashed outline the region illustrated in close-up to the right. The lattices at the top and centre are viewed from outside; the lattice at the bottom is cut away at the front, and the indicated hub is viewed from the inside. (B) Close-up view, in surface rendering, of the hub indicated in (A). The triskelion centred at the vertex shown is in orange; triskelions centred at nearest-neighbour vertices are in yellow; triskelions centred at second nearest-neighbour vertices are in light blue and triskelions centred at third nearest-neighbour vertices are in dark blue. The auxilin fragment, outlined in red, lies between the dark blue terminal domains and the light blue ankle segments of clathrin. Hsc70, in green, binds in the funnel-like cavity bounded by these segments. The clathrin chains are in surface rendering from the molecular model; the auxilin and Hsc70 are in basket contours, based on the density.
Figure 6
Conformational changes in clathrin that accompany binding of auxilin and Hsc70. (A) Axial ratios of D6 coats. The height (H) and two equatorial widths (W1 and W2), illustrated in the cartoon, are the distances between corresponding pairs of atoms at the outer margins of the molecular models. (B) Local changes in the conformation of the N-terminal parts of a triskelion in response to binding of auxilin (green) or auxilin plus Hsc70 (red). The reference triskelion is in blue. (C) Density maps and ribbon representations of a single triskelion leg from the unliganded coat (blue) and the auxilin:Hsc70-bound coat (red). Superposition determined at the hub of the triskelion, as in (B). Top: complete leg; bottom: detail of N-terminal region. The maps have been contoured generously, to show clearly the lower density of the terminal domain and linker; hence, the relatively ‘loose' fit of the proximal and distal legs.
Figure 7
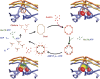
Model for the uncoating mechanism. The central diagram is a schematic representation of the underlying Hsc70/clathrin cycle, and the four corner diagrams show details of binding events at a vertex. Clockwise, from upper left: clathrin coat binds auxilin (red), which stabilizes a strained clathrin conformation (manifested by change in axial ratio of coat); auxilin recruits Hsc70:ATP (ATPase domain in yellow; substrate-binding domain in green); Hsc70 cleaves ATP and substrate-binding domain clamps tightly onto a specific segment of the disordered C-terminal tail of the heavy chain, trapping further strain in the clathrin lattice; when a large enough number of vertices have bound Hsc70, the accumulated strain causes the coat to dissociate, releasing auxilin, clathrin:Hsc70:ADP and Pi. Nucleotide exchange and dissociation of Hsc70 from clathrin complete the cycle.
Similar articles
- Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating.
Fotin A, Cheng Y, Grigorieff N, Walz T, Harrison SC, Kirchhausen T. Fotin A, et al. Nature. 2004 Dec 2;432(7017):649-53. doi: 10.1038/nature03078. Epub 2004 Oct 24. Nature. 2004. PMID: 15502813 - A sequential mechanism for clathrin cage disassembly by 70-kDa heat-shock cognate protein (Hsc70) and auxilin.
Rothnie A, Clarke AR, Kuzmic P, Cameron A, Smith CJ. Rothnie A, et al. Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6927-32. doi: 10.1073/pnas.1018845108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482805 Free PMC article. - A motif in the clathrin heavy chain required for the Hsc70/auxilin uncoating reaction.
Rapoport I, Boll W, Yu A, Böcking T, Kirchhausen T. Rapoport I, et al. Mol Biol Cell. 2008 Jan;19(1):405-13. doi: 10.1091/mbc.e07-09-0870. Epub 2007 Oct 31. Mol Biol Cell. 2008. PMID: 17978091 Free PMC article. - Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis.
Eisenberg E, Greene LE. Eisenberg E, et al. Traffic. 2007 Jun;8(6):640-6. doi: 10.1111/j.1600-0854.2007.00568.x. Epub 2007 May 4. Traffic. 2007. PMID: 17488288 Review. - The role of molecular chaperones in clathrin mediated vesicular trafficking.
Sousa R, Lafer EM. Sousa R, et al. Front Mol Biosci. 2015 May 19;2:26. doi: 10.3389/fmolb.2015.00026. eCollection 2015. Front Mol Biosci. 2015. PMID: 26042225 Free PMC article. Review.
Cited by
- Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in Saccharomyces cerevisiae.
Walters RW, Muhlrad D, Garcia J, Parker R. Walters RW, et al. RNA. 2015 Sep;21(9):1660-71. doi: 10.1261/rna.053116.115. Epub 2015 Jul 21. RNA. 2015. PMID: 26199455 Free PMC article. - Hsp70 May Be a Molecular Regulator of Schistosome Host Invasion.
Ishida K, Jolly ER. Ishida K, et al. PLoS Negl Trop Dis. 2016 Sep 9;10(9):e0004986. doi: 10.1371/journal.pntd.0004986. eCollection 2016 Sep. PLoS Negl Trop Dis. 2016. PMID: 27611863 Free PMC article. - Adenovirus flow in host cell networks.
Flatt JW, Butcher SJ. Flatt JW, et al. Open Biol. 2019 Feb 28;9(2):190012. doi: 10.1098/rsob.190012. Open Biol. 2019. PMID: 30958097 Free PMC article. Review. - J-domain protein chaperone circuits in proteostasis and disease.
Zhang R, Malinverni D, Cyr DM, Rios PL, Nillegoda NB. Zhang R, et al. Trends Cell Biol. 2023 Jan;33(1):30-47. doi: 10.1016/j.tcb.2022.05.004. Epub 2022 Jun 18. Trends Cell Biol. 2023. PMID: 35729039 Free PMC article. Review. - Synthesis of a 3,7-Disubstituted Isothiazolo[4,3-b]pyridine as a Potential Inhibitor of Cyclin G-Associated Kinase.
Grisez T, Ravi NP, Froeyen M, Schols D, Van Meervelt L, De Jonghe S, Dehaen W. Grisez T, et al. Molecules. 2024 Feb 22;29(5):954. doi: 10.3390/molecules29050954. Molecules. 2024. PMID: 38474466 Free PMC article.
References
- Alfano C, McMacken R (1989) Heat shock protein-mediated disassembly of nucleoprotein structures is required for the initiation of bacteriophage lambda DNA replication. J Biol Chem 264: 10709–10718 - PubMed
- Anderson RG, Brown MS, Goldstein JL (1977) Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell 10: 351–364 - PubMed
- Barouch W, Prasad K, Greene L, Eisenberg E (1997) Auxilin-induced interaction of the molecular chaperone Hsc70 with clathrin baskets. Biochemistry 36: 4303–4308 - PubMed
- Barouch W, Prasad K, Greene LE, Eisenberg E (1994) ATPase activity associated with the uncoating of clathrin baskets by Hsp70. J Biol Chem 269: 28563–28568 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM036548/GM/NIGMS NIH HHS/United States
- P01 GM062580/GM/NIGMS NIH HHS/United States
- GM-36548/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- GM-62580/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous