Electron transport chain dysfunction in neonatal pressure-overload hypertrophy precedes cardiomyocyte apoptosis independent of oxidative stress - PubMed (original) (raw)
Electron transport chain dysfunction in neonatal pressure-overload hypertrophy precedes cardiomyocyte apoptosis independent of oxidative stress
Eric R Griffiths et al. J Thorac Cardiovasc Surg. 2010 Jun.
Abstract
Objectives: We have previously shown in a model of pressure-overload hypertrophy that there is increased cardiomyocyte apoptosis during the transition from peak hypertrophy to ventricular decompensation. Electron transport chain dysfunction is believed to play a role in this process through the production of excessive reactive oxygen species. In this study we sought to determine electron transport chain function in pressure-overload hypertrophy and the role of oxidative stress in myocyte apoptosis.
Methods and results: Neonatal rabbits underwent thoracic aortic banding at 10 days of age. Compensated hypertrophy (4 weeks of age), decompensated hypertrophy (6 weeks of age), and age-matched controls (n = 4-8 per group) as identified by serial echocardiography were studied. Electron transport chain complex activities were determined by spectophotometry in isolated mitochondria. Complex I was significantly decreased (P = .005) at 4 weeks and further decreased at 6 weeks (P = .001). Complex II was significantly decreased at both time points (4 weeks, P = .003; 6 weeks, P = .009). However, hyddrogen peroxide production, measured in isolated mitochondria by fluorescence spectroscopy, was significantly decreased at 4 weeks of age in banded animals compared with controls (P = .038), and mitochondrial DNA oxidative damage (measurement of 8- hydroxydeoxyguanosine by enzyme-linked immunosorbent assay) was also significantly decreased at 4 weeks of age (P = .031). Mitochondrial activated apoptosis was determined by Bax/Bcl-2 ratios (immunoblotting). Bax/Bcl-2 levels were significantly increased in banded animals at 6 weeks.
Conclusions: In pressure-overload hypertrophy, the transition from compensated left ventricular hypertrophy to failure and cardiomyocyte apoptosis is preceded by mitochondrial complex I and II dysfunction followed by an increase in Bax/Bcl-2 ratios. The mechanism of apoptosis initiation is independent of increased oxidative stress.
Copyright 2010 The American Association for Thoracic Surgery. Published by Mosby, Inc. All rights reserved.
Figures
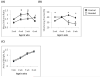
Figure 1
Transthoracic echocardiography showing development of LV hypertrophy and later decompensation. (A) Increase in Mass/Volume (M/V) ratio indicating peak hypertrophy in the banded animals occurred at 4–5 wks of age followed by ventricular dilatation at 6wks of age evidenced by a decrease in M/V ratio. (B) Contractile performance was assessed by fractional shortening (FS). The FS was preserved during hypertrophy at 4wks of age and decreased as the ventricle dilated at 6wks (n=6–11, * = p<0.05). (C) There was no significant difference in animal body weights. Banded animals at 6wks of age had no clinical signs of heart failure such as ascites or pleural effusions.
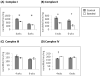
Figure 2
ETC Complex Activities measured by spectophotometry in isolated mitochondria from hypertrophied (4wks) and decompensated hypertrophy (6wks) LV myocardium. Complex I (A) and Complex II (B) activities were significantly decreased at 4 wks of age and further decreased significantly at 6wks of age. (c) Complex III activity was unchanged. (D) Complex IV activity was significantly increased at 4wks of age but normalizes at 6wks of age.(n =4,* p<0.005, †p=0.009, ‡ p= 0.03)
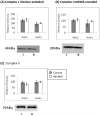
Figure 3
Western blot analysis of ETC complex subunit protein levels at 4wks and 6wks of age. Representative blots are shown. Nuclear encoded, 30kDa Iron-Sulfur Protein 3 (A) and the mitochondrial encoded 20 kDa, ND6 subunits (B) of complex I are unchanged at both time points. (C) The protein level of the complex II 70 kDa flavoprotein is unchanged change as well. Representative immunobots are shown and bar graphs depict cumulative data which are expressed are as means ± SEM (n=4 *p≤0.05).
Figure 4
Determination of ROS generation and oxidative damage. Production of H202 was determined by fluorescence spectroscopy using the Amplex Red reaction in isolated mitochondria. (A) mitochondria at 4wks of age (hypertrophy) showed significantly decreased H202 production. (B)mitochondria at 6wks of age (decompenated hypertrophy) show decreased, but not significantly, production of H202. 8-OHdG, as a marker for oxidative DNA damage was measured in digested nuclear and mitochondrial DNA. There was no difference in 8-OHdG levels in nuclear DNA (C) at either time point. In mtDNA (D) 8-OHdG levels were significantly decreased at 4 wks of age (Data expressed are as means ± SEM, n=4-6, *p≤0.05).
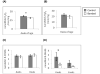
Figure 5
Summarized results of TUNEL staining for cardiomyocyte apoptosis at 4wks and 6wks of age in aortic banded and control animals. There was a nonsignificant increase in cardiomyocyte apoptosis at 4wks of age compared to controls and a significant increase in banded animals at 6wks of age compared to controls. Results are expressed as means + std (n=3) of TUNEL positive cardiomyocyte nuclei per 1000 nuclei, * p =.025.
Figure 6
Western blot analysis of Bax and Bcl-2 protein levels at 6wks of age when cardiomycyte apoptosis is known to be significantly increased. (A) Significantly increased Bax protein levels in banded animals and (B) significantly increased Bax/Bcl-2 ratio. Representative immunobots are shown and bar graphs depict cumulative data which are expressed are as means ± SEM (n=4 *p≤0.05).
Similar articles
- EGCG inhibits cardiomyocyte apoptosis in pressure overload-induced cardiac hypertrophy and protects cardiomyocytes from oxidative stress in rats.
Sheng R, Gu ZL, Xie ML, Zhou WX, Guo CY. Sheng R, et al. Acta Pharmacol Sin. 2007 Feb;28(2):191-201. doi: 10.1111/j.1745-7254.2007.00495.x. Acta Pharmacol Sin. 2007. PMID: 17241521 - Overexpression of miR-142-3p improves mitochondrial function in cardiac hypertrophy.
Liu BL, Cheng M, Hu S, Wang S, Wang L, Tu X, Huang CX, Jiang H, Wu G. Liu BL, et al. Biomed Pharmacother. 2018 Dec;108:1347-1356. doi: 10.1016/j.biopha.2018.09.146. Epub 2018 Oct 4. Biomed Pharmacother. 2018. PMID: 30372837 - Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure.
Li XM, Ma YT, Yang YN, Liu F, Chen BD, Han W, Zhang JF, Gao XM. Li XM, et al. Clin Exp Pharmacol Physiol. 2009 Nov;36(11):1054-61. doi: 10.1111/j.1440-1681.2009.05243.x. Epub 2009 Jun 29. Clin Exp Pharmacol Physiol. 2009. PMID: 19566828 - Alterations in mitochondrial function in cardiac hypertrophy and heart failure.
Osterholt M, Nguyen TD, Schwarzer M, Doenst T. Osterholt M, et al. Heart Fail Rev. 2013 Sep;18(5):645-56. doi: 10.1007/s10741-012-9346-7. Heart Fail Rev. 2013. PMID: 22968404 Review. - Mitochondria and Cardiac Hypertrophy.
Facundo HDTF, Brainard RE, Caldas FRL, Lucas AMB. Facundo HDTF, et al. Adv Exp Med Biol. 2017;982:203-226. doi: 10.1007/978-3-319-55330-6_11. Adv Exp Med Biol. 2017. PMID: 28551789 Review.
Cited by
- Sustained Oligomycin Sensitivity Conferring Protein Expression in Cardiomyocytes Protects Against Cardiac hypertrophy Induced by Pressure Overload via Improving Mitochondrial Function.
Guo Y, Zhang K, Gao X, Zhou Z, Liu Z, Yang K, Huang K, Yang Q, Long Q. Guo Y, et al. Hum Gene Ther. 2020 Nov;31(21-22):1178-1189. doi: 10.1089/hum.2020.004. Epub 2020 Sep 21. Hum Gene Ther. 2020. PMID: 32787458 Free PMC article. - Study of respiratory chain dysfunction in heart disease.
Hassanpour SH, Dehghani MA, Karami SZ. Hassanpour SH, et al. J Cardiovasc Thorac Res. 2018;10(1):1-13. doi: 10.15171/jcvtr.2018.01. Epub 2018 Mar 17. J Cardiovasc Thorac Res. 2018. PMID: 29707171 Free PMC article. Review. - Mitophagy: A Potential Target for Pressure Overload-Induced Cardiac Remodelling.
Shao R, Li J, Qu T, Liao Y, Chen M. Shao R, et al. Oxid Med Cell Longev. 2022 Sep 27;2022:2849985. doi: 10.1155/2022/2849985. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36204518 Free PMC article. Review. - Selective downregulation of mitochondrial electron transport chain activity and increased oxidative stress in human atrial fibrillation.
Emelyanova L, Ashary Z, Cosic M, Negmadjanov U, Ross G, Rizvi F, Olet S, Kress D, Sra J, Tajik AJ, Holmuhamedov EL, Shi Y, Jahangir A. Emelyanova L, et al. Am J Physiol Heart Circ Physiol. 2016 Jul 1;311(1):H54-63. doi: 10.1152/ajpheart.00699.2015. Epub 2016 May 6. Am J Physiol Heart Circ Physiol. 2016. PMID: 27199126 Free PMC article. - Monoamine Oxidases as Potential Contributors to Oxidative Stress in Diabetes: Time for a Study in Patients Undergoing Heart Surgery.
Duicu OM, Lighezan R, Sturza A, Ceausu RA, Borza C, Vaduva A, Noveanu L, Gaspar M, Ionac A, Feier H, Muntean DM, Mornos C. Duicu OM, et al. Biomed Res Int. 2015;2015:515437. doi: 10.1155/2015/515437. Epub 2015 May 25. Biomed Res Int. 2015. PMID: 26101773 Free PMC article. Review.
References
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annual review of physiology. 2003;65:45–79. - PubMed
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological reviews. 2005;85(3):1093–1129. - PubMed
- Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. Journal of the American College of Cardiology. 2002;40(7):1267–1274. - PubMed
Publication types
MeSH terms
Grants and funding
- K08 HL075430/HL/NHLBI NIH HHS/United States
- T32 HL007734/HL/NHLBI NIH HHS/United States
- R01 HL063095/HL/NHLBI NIH HHS/United States
- HL-063095/HL/NHLBI NIH HHS/United States
- HL-075430/HL/NHLBI NIH HHS/United States
- HL-074734/HL/NHLBI NIH HHS/United States
- R01 HL066186/HL/NHLBI NIH HHS/United States
- R01 HL063095-09/HL/NHLBI NIH HHS/United States
- HL-0066186/HL/NHLBI NIH HHS/United States
- T32 HL 007734/HL/NHLBI NIH HHS/United States
- P50 HL074734/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials