Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4 - PubMed (original) (raw)
Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4
Michelle L Janas et al. J Exp Med. 2010.
Abstract
T cell development requires phosphatidylinositol 3-kinase (PI3K) signaling with contributions from both the class IA, p110delta, and class IB, p110gamma catalytic subunits. However, the receptors on immature T cells by which each of these PI3Ks are activated have not been identified, nor has the mechanism behind their functional redundancy in the thymus. Here, we show that PI3K signaling from the preTCR requires p110delta, but not p110gamma. Mice deficient for the class IB regulatory subunit p101 demonstrated the requirement for p101 in T cell development, implicating G protein-coupled receptor signaling in beta-selection. We found evidence of a role for CXCR4 using small molecule antagonists in an in vitro model of beta-selection and demonstrated a requirement for CXCR4 during thymic development in CXCR4-deficient embryos. Finally, we demonstrate that CXCL12, the ligand for CXCR4, allows for Notch-dependent differentiation of DN3 thymocytes in the absence of supporting stromal cells. These findings establish a role for CXCR4-mediated PI3K signaling that, together with signals from Notch and the preTCR, contributes to continued T cell development beyond beta-selection.
Figures
Figure 1.
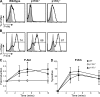
PI3K p110δ is required for preTCR signaling in DN4 thymocytes. (A) Phosphorylated Akt (S473) and (B) phosphorylated Erk1/2 (T202/Y204) in WT and PI3K mutant DN4 T cells in response to preTCR signaling. Histograms show staining in either resting cells (gray area) or cells stimulated with 10 µg/ml of anti-CD3ε antibody 2C11 for 5 min (black line). DN4 cells were derived from long-term BM HSC cultures. Intracellular p-Akt and p-Erk1/2 were detected using flow cytometry in combination with cell surface markers to distinguish the DN4 population (defined as Thy1.2+ and [CD4/CD8/CD19/CD25]neg). (C and D) Time course of p-Akt (S473) and pErk induction in WT, p110δ−/−, and p110γ−/− cultured DN4 cells in response to stimulation with 2C11. Graphs show the mean and SD (n = 3–5) and are comprised of five independent experiments.
Figure 2.
Defective maturation of DN3 thymocytes in mice that lack p110δ in combination with either p110γ or p101. (A) Flow cytometric analysis of CD4 and CD8 expression on T lymphocytes in the thymus from WT and PI3K mutant mice. The percentages of CD4+CD8+ DP and CD4−CD8− DN cells are shown. The mean thymus cellularity + SEM for each genotype is also shown (n = 9–33). (B) Flow cytometric analysis of the DN3/DN4 thymic subsets in PI3K mutant mice (identified as Thy1+ and [CD4/CD8/CD44/B220/CD11b/ NK1.1/Gr1/Ter119/γδTCR]neg). The percentages of CD25loCD98hi, CD25loCD71hi, icTCRβ−, icTCRβ+FSClo, and icTCRβ+FSChi are shown. Enumeration of CD25loCD98hi and CD25loCD71hi DN3/DN4 cells (C) and numbers of icTCR+ FSClo and FSChi cells (D) for each genotype are shown. In C, graphs represent the mean and SD (n = 3–5). In D, graphs show the mean and SEM (n = 5–27).
Figure 3.
T lymphocyte development in BM chimeras. (A) CD4 and CD8 expression on thymocytes with the percentages of DP and DN cells shown. The average thymus cellularity + SEM for each group is shown (n = 15–20). (B) Expression of CD25 and CD44 on DN thymocytes with the percentages of CD44−CD25+ and CD44−CD25− cells shown. (C) Enumeration of CD44−CD25+ and CD44−CD25− and CD4+CD8+ DP thymocytes in chimeras reconstituted with WT, p110γ−/−δ−/−, and p101−/−/p110δ−/− marrow. Graphs represent the mean and SEM. n = 15–19. (D) Flow cytometric analysis of the DN3/DN4 thymic subset. The percentages of CD25loCD98hi, CD25loCD71hi, icTCRβ−, icTCRβ+FSClo, and icTCRβ+FSChi are shown. Plots and percentages are representative of at least five individual mice. Enumeration of CD25loCD98hi and CD25loCD71hi cells (E) and icTCR+FSClo and FSChi cells (F) for each genotype are shown. (G and H) Analysis of DN3/DN4 cell proliferation by EdU incorporation. Graphs show the mean and SD from analysis of five independent mice.
Figure 4.
DN3 cells from p110γ−/−δ−/− and p101−/−/p110δ−/− mice fail to differentiate in vitro. 4 × 104 DN3 cells from WT and PI3K mutant mice were cultured on OP9-DL1 stroma for 3 d. (A) The percentage of CD4+CD8+ cells. (B) The percentage of CD98+ cells. (C) The number of total CD4+CD8+ and CD4−CD8− cells recovered. Graphs show each biological sample (black diamond) as well as the mean (black bars) for each group. This experiment is representative of three independent replicates.
Figure 5.
Inhibition of CXCR4 signaling blocks DN3 cell differentiation. 4 × 104 DN3 cells from WT mice were cultured on OP9-DL1 stromal cells for 3 d in the presence of increasing concentrations of AMD3100 or GSK812397A. Cells were harvested, analyzed for the expression of CD4 and CD8 (A), and absolute numbers of CD4+CD8+ DP and CD4−CD8− DN cells recovered (B). Graphs show the mean and SD. n = 3. This experiment is representative of three independent replicates.
Figure 6.
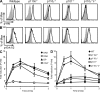
PI3K p110γ and p110δ are required for CXCR4 signaling in DN3 thymocytes. (A) Phosphorylated Akt (S473) or (B) phosphorylated Erk1/2 (T202/Y204) in WT and PI3K mutant DN3 thymocytes after stimulation with CXCL12. Histograms show staining of resting cells or cells stimulated with 10 nM of CXCL12 for either 1 min (p-Akt) or 2 min (p-Erk1/2). Single-cell suspensions of whole thymus were used for stimulation. Intracellular p-Akt (S473) and p-Erk1/2 was detected using flow cytometry in combination with cell surface markers to distinguish the DN3 population. (C) Time course of p-Akt (S473) induction in WT DN3, DN4, DP, and CD4+SP thymocytes. (D) Time course of p-Akt (S473) induction in WT, p110δ−/−, p110γ−/−, p101−/−, and p110γ−/−δ−/− DN3 thymocytes. Data are presented as fold induction, which is calculated as the median fluorescence of phosphorylated protein in stimulated/unstimulated cells. Each data point shows the mean and SD of three to four biological replicates and is comprised from two independent experiments.
Figure 7.
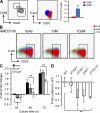
PI3K activity increases during β-selection. (A) CD98lo (red) and CD98hi (blue) DN3 thymocytes were sort purified from WT mice before fixation, permeabilization, and intracellular staining for p-Akt (S473). The fold change in p-Akt was calculated as the median fluorescence of p-Akt in CD98hi compared with CD98lo cells. (B and C) 4 × 104 DN3 cells from WT mice were cultured on OP9-DL1 stromal cells for 3 d in the presence of increasing concentrations of AMD3100. At 24 (red), 48 (blue), and 72 h (green) cells were harvested and analyzed for the expression of p-Akt. The fold change in p-Akt levels was measured relative to cells harvested at 24 h that had been cultured in the absence of AMD3100. Results presented in A–C are from a single experiment of three biological replicates (with each biological sample comprising a pool of sorted cells from five mice). (D) p-Akt (S473) expression in DN3 cells isolated ex vivo from PI3K mutant mice. Data are presented as p-Akt expression relative to WT and 4–11 mice were analyzed for each genotype. All graphs show the mean and SD.
Figure 8.
Embryonic thymic development is perturbed in CXCR4-deficient mice. Thymocyte composition fin E17.5 CXCR4+/− or CXCR4−/− embryos (A, top). Flow cytometric analysis of CD4 and CD8 expression. The percentages of CD4+CD8+ (DP) and CD4−CD8− (DN) cells are shown. (A, bottom) Flow cytometric analysis CD25 and CD44 expression on CD3ε/CD4/CD8neg thymocytes. The percentages of CD44hiCD25lo (DN1), CD44hiCD25hi (DN2), CD44loCD25hi (DN3), and CD44l°CD25lo (DN4) are shown. (B and C) The number of DN1, DN2, DN3, DN4, and DP thymocytes. For subset analysis, the ratio of CXCR4−/− to CXCR4+/− numbers is shown. (D) Comparison of CXCR4+/− and CXCR4−/− DN3 and DN4 cell size based on forward light scatter analysis. Size is represented relative to CXCR4+/−. All graphs represent the mean and SD (n = 3).
Figure 9.
Establishment of an accessory cell–free culture system for the differentiation of immature thymocytes. (A–C) 105 DN3 cells from WT mice were cultured on OP9-DL1 or OP9 stromal cells, plate-bound recombinant Delta-like 4 (rDL4), CXCL12, or rDL4 and either CXCL12, CCL5, or CCL25. After 3 d, cells were harvested and analyzed for the percentage of CD4+CD8+, CD4−CD8− (A) cells and the percentage of CD98+ cells (B). (C) The total number of cells harvested under different culture conditions. The graph shows the mean and SEM of five biological replicates, except for cells cultured with CXCL12 alone, where n = 1. (D and E) 105 DN3 cells from WT or p110γ−/−δ−/− mice were cultured with rDL4 with 10 µM CHIR99021 in the presence or absence of 10nM CXCL12. After 3 d, cells were harvested and analyzed for the percentages of CD4+CD8+ and CD4−CD8− cells (D) and the total number of cells harvested (E). The graph shows the mean and SD of two biological replicates. Results from WT represent one of three experiments.
Similar articles
- RasGRP1, but not RasGRP3, is required for efficient thymic β-selection and ERK activation downstream of CXCR4.
Golec DP, Dower NA, Stone JC, Baldwin TA. Golec DP, et al. PLoS One. 2013;8(1):e53300. doi: 10.1371/journal.pone.0053300. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308188 Free PMC article. - Costimulatory role of CXCR4 with pre-TCR and its crosstalk with PI3K in beta-selection of thymocytes.
Ahamed JA, Madhivadhani P. Ahamed JA, et al. Sci Signal. 2010 Apr 27;3(119):jc4. doi: 10.1126/scisignal.3119jc4. Sci Signal. 2010. PMID: 20424260 - Stromal cell-derived factor 1α and CXCR4: newly defined requirements for efficient thymic β-selection.
Janas ML, Turner M. Janas ML, et al. Trends Immunol. 2010 Oct;31(10):370-6. doi: 10.1016/j.it.2010.07.002. Epub 2010 Sep 9. Trends Immunol. 2010. PMID: 20829112 Review. - CXCR4 acts as a costimulator during thymic beta-selection.
Trampont PC, Tosello-Trampont AC, Shen Y, Duley AK, Sutherland AE, Bender TP, Littman DR, Ravichandran KS. Trampont PC, et al. Nat Immunol. 2010 Feb;11(2):162-70. doi: 10.1038/ni.1830. Epub 2009 Dec 13. Nat Immunol. 2010. PMID: 20010845 Free PMC article.
Cited by
- Critical role of WNK1 in MYC-dependent early mouse thymocyte development.
Köchl R, Vanes L, Llorian Sopena M, Chakravarty P, Hartweger H, Fountain K, White A, Cowan J, Anderson G, Tybulewicz VL. Köchl R, et al. Elife. 2020 Oct 14;9:e56934. doi: 10.7554/eLife.56934. Elife. 2020. PMID: 33051000 Free PMC article. - PI3Ks in lymphocyte signaling and development.
Okkenhaug K, Fruman DA. Okkenhaug K, et al. Curr Top Microbiol Immunol. 2010;346:57-85. doi: 10.1007/82_2010_45. Curr Top Microbiol Immunol. 2010. PMID: 20563708 Free PMC article. Review. - αβ versus γδ fate choice: counting the T-cell lineages at the branch point.
Kreslavsky T, Gleimer M, Garbe AI, von Boehmer H. Kreslavsky T, et al. Immunol Rev. 2010 Nov;238(1):169-81. doi: 10.1111/j.1600-065X.2010.00947.x. Immunol Rev. 2010. PMID: 20969592 Free PMC article. Review. - Adaptive Immunodeficiency in WHIM Syndrome.
Majumdar S, Murphy PM. Majumdar S, et al. Int J Mol Sci. 2018 Dec 20;20(1):3. doi: 10.3390/ijms20010003. Int J Mol Sci. 2018. PMID: 30577453 Free PMC article. Review. - Multiple levels of chemokine receptor regulation in the control of mouse natural killer cell development.
Bernardini G, Benigni G, Antonangeli F, Ponzetta A, Santoni A. Bernardini G, et al. Front Immunol. 2014 Feb 13;5:44. doi: 10.3389/fimmu.2014.00044. eCollection 2014. Front Immunol. 2014. PMID: 24592263 Free PMC article. Review.
References
- Amara A., Lorthioir O., Valenzuela A., Magerus A., Thelen M., Montes M., Virelizier J.L., Delepierre M., Baleux F., Lortat-Jacob H., Arenzana-Seisdedos F. 1999. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J. Biol. Chem. 274:23916–23925 10.1074/jbc.274.34.23916 - DOI - PubMed
- Ara T., Itoi M., Kawabata K., Egawa T., Tokoyoda K., Sugiyama T., Fujii N., Amagai T., Nagasawa T. 2003. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J. Immunol. 170:4649–4655 - PubMed
- Callard R., Hodgkin P. 2007. Modeling T- and B-cell growth and differentiation. Immunol. Rev. 216:119–129 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases