Role of A-type lamins in signaling, transcription, and chromatin organization - PubMed (original) (raw)
Review
Role of A-type lamins in signaling, transcription, and chromatin organization
Vicente Andrés et al. J Cell Biol. 2009.
Abstract
A-type lamins (lamins A and C), encoded by the LMNA gene, are major protein constituents of the mammalian nuclear lamina, a complex structure that acts as a scaffold for protein complexes that regulate nuclear structure and functions. Interest in these proteins has increased in recent years with the discovery that LMNA mutations cause a variety of human diseases termed laminopathies, including progeroid syndromes and disorders that primarily affect striated muscle, adipose, bone, and neuronal tissues. In this review, we discuss recent research supporting the concept that lamin A/C and associated nuclear envelope proteins regulate gene expression in health and disease through interplay with signal transduction pathways, transcription factors, and chromatin-associated proteins.
Figures
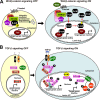
Figure 1.
Control of Wnt–β-catenin and TGF-β signaling by A-type lamins and associated proteins. (A, left) In the absence of Wnt, cytoplasmic β-catenin (β-cat) undergoes proteasomal degradation, and TCF-dependent transcription is repressed. (right) Upon Wnt binding to its receptors (e.g., frizzled), β-catenin accumulates in the cytoplasm, translocates to the nucleus, and induces TCF-dependent transcription. Emerin exports nuclear β-catenin to the cytoplasm, thereby inhibiting TCF-dependent transcription. GSK3β, glycogen synthase kinase 3β. (B, left) In the absence of TGF-β, hypophosphorylated R-Smads accumulate in the cytoplasm. (right) Binding of TGF-β to its receptors leads to R-Smad phosphorylation and the formation and nuclear import of R-Smad–Smad4 complexes, which induce target gene expression in conjunction with other transcription factors (TF). MAN1 might sequester active R-Smads at the NE, preventing them from oligomerizing with co-Smads. A-type lamins interact with activated PP2A, which can dephosphorylate R-Smads and ppRb.
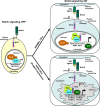
Figure 2.
Control of Notch signaling by lamin A/C and progerin. (left) In the absence of Notch ligands, transcription of target genes is repressed. (right) Notch activation by neighboring cells leads to proteolytic release of the NICD, which translocates to the nucleus, where it activates target genes upon binding to coactivators and the release of corepressors (Co-R) from the promoter. Stem cells from HGPS patients express progerin. Notch signaling is elevated in these cells, and there is an up-regulation in the expression of Notch target genes, coincident with reduced amounts of the repressor NcoR and increased availability of the coactivator SKIP in the nuclear interior. Mam, Mastermind; DSL, Delta/Serrate/LAG-2 family of proteins.
Figure 3.
Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the NE. (left) Quiescent cells contain low levels of c-Fos, which is predominantly hypophosphorylated and sequestered at the NE through its interaction with A-type lamins. (right) Upon mitogen stimulation, phosphorylated (active) ERKs 1 and 2 interact with A-type lamins and phosphorylate c-Fos, releasing it from the NE. The released c-Fos can heterodimerize in the nucleoplasm with other AP-1 family members (e.g., c-Jun), allowing the activation of AP-1 target genes before de novo c-Fos synthesis.
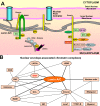
Figure 4.
Interactions of A-type lamins and NE-associated proteins with DNA, chromatin complexes, and related transcription factors. (A) Schematic illustration of the mammalian NE showing proteins involved in the organization and regulation of chromatin and gene expression. (B) Network of NE-associated chromatin complexes. The thick lines indicate direct interactions with lamin A/C. CRX, cone-rod homeobox.
Similar articles
- [Research progress in A-type lamins].
Gao CH, Liu XG, Zhou ZJ. Gao CH, et al. Sheng Li Ke Xue Jin Zhan. 2009 Jul;40(3):203-8. Sheng Li Ke Xue Jin Zhan. 2009. PMID: 19803422 Review. Chinese. - Lamina-associated polypeptide (LAP)2α and nucleoplasmic lamins in adult stem cell regulation and disease.
Gesson K, Vidak S, Foisner R. Gesson K, et al. Semin Cell Dev Biol. 2014 May;29(100):116-24. doi: 10.1016/j.semcdb.2013.12.009. Epub 2013 Dec 25. Semin Cell Dev Biol. 2014. PMID: 24374133 Free PMC article. Review. - [Nuclear lamins regulate osteogenic differentiation of mesenchymal stem cells].
Bogdanova MA, Gudkova AIa, Zabirnik AS, Ignat'eva EV, Dmitrieva RI, Smolina NA, Kostareva AA, Malashicheva AB. Bogdanova MA, et al. Tsitologiia. 2014;56(4):260-7. Tsitologiia. 2014. PMID: 25509159 Russian. - Mutations in LMNA modulate the lamin A--Nesprin-2 interaction and cause LINC complex alterations.
Yang L, Munck M, Swaminathan K, Kapinos LE, Noegel AA, Neumann S. Yang L, et al. PLoS One. 2013 Aug 20;8(8):e71850. doi: 10.1371/journal.pone.0071850. eCollection 2013. PLoS One. 2013. PMID: 23977161 Free PMC article. - Suppression of myopathic lamin mutations by muscle-specific activation of AMPK and modulation of downstream signaling.
Chandran S, Suggs JA, Wang BJ, Han A, Bhide S, Cryderman DE, Moore SA, Bernstein SI, Wallrath LL, Melkani GC. Chandran S, et al. Hum Mol Genet. 2019 Feb 1;28(3):351-371. doi: 10.1093/hmg/ddy332. Hum Mol Genet. 2019. PMID: 30239736 Free PMC article.
Cited by
- Cell-Matrix Interactions and Matricrine Signaling in the Pathogenesis of Vascular Calcification.
Ngai D, Lino M, Bendeck MP. Ngai D, et al. Front Cardiovasc Med. 2018 Dec 7;5:174. doi: 10.3389/fcvm.2018.00174. eCollection 2018. Front Cardiovasc Med. 2018. PMID: 30581820 Free PMC article. Review. - Subcellular localization of SREBP1 depends on its interaction with the C-terminal region of wild-type and disease related A-type lamins.
Duband-Goulet I, Woerner S, Gasparini S, Attanda W, Kondé E, Tellier-Lebègue C, Craescu CT, Gombault A, Roussel P, Vadrot N, Vicart P, Ostlund C, Worman HJ, Zinn-Justin S, Buendia B. Duband-Goulet I, et al. Exp Cell Res. 2011 Dec 10;317(20):2800-13. doi: 10.1016/j.yexcr.2011.09.012. Epub 2011 Oct 4. Exp Cell Res. 2011. PMID: 21993218 Free PMC article. - Proteomic profiling of Myc-associated proteins.
Agrawal P, Yu K, Salomon AR, Sedivy JM. Agrawal P, et al. Cell Cycle. 2010 Dec 15;9(24):4908-21. doi: 10.4161/cc.9.24.14199. Epub 2010 Dec 15. Cell Cycle. 2010. PMID: 21150319 Free PMC article. - Temporal Changes in Nucleus Morphology, Lamin A/C and Histone Methylation During Nanotopography-Induced Neuronal Differentiation of Stem Cells.
Ankam S, Teo BKK, Pohan G, Ho SWL, Lim CK, Yim EKF. Ankam S, et al. Front Bioeng Biotechnol. 2018 May 31;6:69. doi: 10.3389/fbioe.2018.00069. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 29904629 Free PMC article. - The cellular mastermind(?)-mechanotransduction and the nucleus.
Kaminski A, Fedorchak GR, Lammerding J. Kaminski A, et al. Prog Mol Biol Transl Sci. 2014;126:157-203. doi: 10.1016/B978-0-12-394624-9.00007-5. Prog Mol Biol Transl Sci. 2014. PMID: 25081618 Free PMC article. Review.
References
- Bakay M., Wang Z., Melcon G., Schiltz L., Xuan J., Zhao P., Sartorelli V., Seo J., Pegoraro E., Angelini C., et al. 2006. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. 129:996–1013 10.1093/brain/awl023 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous