FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice - PubMed (original) (raw)
. 2010 Jan;120(1):357-68.
doi: 10.1172/JCI39901. Epub 2009 Dec 14.
Affiliations
- PMID: 20038793
- PMCID: PMC2798687
- DOI: 10.1172/JCI39901
FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice
Marie-Therese Rached et al. J Clin Invest. 2010 Jan.
Erratum in
- J Clin Invest. 2010 Mar 1;120(3):932
Abstract
Osteoblasts have recently been found to play a role in regulating glucose metabolism through secretion of osteocalcin. It is unknown, however, how this osteoblast function is regulated transcriptionally. As FoxO1 is a forkhead family transcription factor known to regulate several key aspects of glucose homeostasis, we investigated whether its expression in osteoblasts may contribute to its metabolic functions. Here we show that mice lacking Foxo1 only in osteoblasts had increased pancreatic beta cell proliferation, insulin secretion, and insulin sensitivity. The ability of osteoblast-specific FoxO1 deficiency to affect metabolic homeostasis was due to increased osteocalcin expression and decreased expression of Esp, a gene that encodes a protein responsible for decreasing the bioactivity of osteocalcin. These results indicate that FoxO1 expression in osteoblasts contributes to FoxO1 control of glucose homeostasis and identify FoxO1 as a key modulator of the ability of the skeleton to function as an endocrine organ regulating glucose metabolism.
Figures
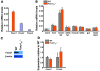
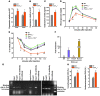
Figure 1. Perinatal lethality in Foxo1ob–/– mice.
(A) Real-time PCR analysis of the expression of the 3 Foxo isoforms in primary osteoblasts; n = 3. (B) Real-time PCR analysis of Foxo1 expression in bone and other tissues of WT and Foxo1ob–/– mice; n = 3 mice/group. Data are presented as mean ± SEM; **P < 0.01 by Student’s t test. (C) Western blot analysis of FoxO1 protein levels in osteoblasts. (D) Real-time PCR analysis of the expression of Foxo3 and Foxo4 in the femur of WT and Foxo1ob–/– mice; n = 3/group. In B and D, mice were 2 months of age.
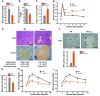
Figure 2. Increased β cell proliferation and insulin secretion in Foxo1ob–/– mice.
(A) Blood glucose levels in WT and Foxo1ob–/– newborn before milk ingestion; n = 5 pups. (B) Blood glucose and (C) serum insulin levels in WT and Foxo1ob–/– mice at random feeding; n = 5. (D) Plasma insulin levels after glucose injection in WT and Foxo1ob–/– mice; n = 4/group. (E) H&E and insulin staining and (F) Ki67 immunostaining showing larger islets and increased β cell proliferation in the pancreas of Foxo1ob–/– mice; n = 5 mice/group. Scale bars represent 100 μm, except in the H&E panels, where they represent 800 μm. (G) Fasting blood glucose levels in WT and Foxo1ob–/– adult mice; n = 5 mice/group. (H) GTT in WT and Foxo1ob–/– mice; n = 5 mice/ group. (I) PTTs in WT and Foxo1ob–/– mice; n = 5 mice/group. In all panels, data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test. In B–I, all mice were 2 months of age.
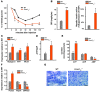
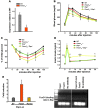
Figure 3. Increased insulin sensitivity in Foxo1ob–/– mice.
(A) ITTs in WT and Foxo1ob–/– mice; n = 5 mice/group. (Identical data are shown in Figure 5E.) (B) Glucose infusion rate (GIR) and suppression of hepatic glucose production (HGP) (% of clamp HGP relative to basal HGP) in WT (n = 3) and Foxo1ob–/– (n = 5) mice by hyperinsulinemic-euglycemic clamps. (C) Real-time PCR analysis of insulin target gene expression in skeletal muscle of WT and Foxo1ob–/– mice. Values are expressed as fold increase relative to WT; n = 4 mice/group. (D and E) HPLC analysis of adenine nucleotide levels in the vastus lateralis muscle; n = 4 mice/group. (F) Real-time PCR analysis of insulin target genes in the liver of WT and Foxo1ob–/– mice; n = 4 mice/group. (G) Oil red O staining in liver sections of WT and Foxo1ob–/– mice; n = 4 mice/group. Scale bars: 100 μm. In all panels, data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test. All mice were 2–3 months of age.
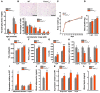
Figure 4. Fat metabolism in Foxo1ob–/– mice.
(A) Gonadal fat pad weight in WT and Foxo1ob–/– mice; n = 7 mice/group. (B) Histomorphometric analysis of white fat sections of WT and Foxo1ob–/– mice; n = 5 mice/group. Scale bars: 400 μm. (C) Body weight curve analysis of WT and Foxo1ob–/– mice from day 5 until 6 months of age; n = 7 mice/group. (D–G) Heat production, oxygen consumption, CO2 expenditure, and total activity (counts) by indirect calorimetric analysis in adult WT and Foxo1ob–/– mice; n = 6 mice/group. (H and I) Real-time PCR analysis of insulin target genes in white fat of WT and Foxo1ob–/– mice; n = 4 mice/group. (J) Serum adiponectin levels in WT and Foxo1ob–/– mice; n = 5 mice/group. (K) Real-time PCR analysis of adiponectin target genes in skeletal muscle of WT and Foxo1ob–/– mice; n = 5 mice/group. (L) Serum leptin levels in WT and Foxo1ob–/– mice; n = 4 mice/group. In all panels, bars indicate mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test. In all panels except C, mice were 2–3 months of age.
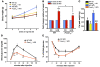
Figure 5. Foxo1 in osteoblasts regulates glucose homeostasis through regulating osteocalcin.
(A) Expression analysis of the 2 insulin genes (Ins1 and Ins2) in WT islets cultured with WT or Foxo1ob–/– primary osteoblasts. (B) Real-time PCR analysis of osteocalcin expression in WT and Foxo1ob–/– bones; n = 3 mice/group. (C) Serum osteocalcin levels in WT and Foxo1ob–/– mice; n = 5 mice/group. (D) GTT in WT and Foxo1ob–/–Ocn+/– mice; n = 5 mice/group. (E) ITT in WT and Foxo1ob–/–Ocn+/– mice; n = 5 mice/group. (F) Cotransfection analysis of FoxO1 interaction with the Ocn promoter in Cos-7 cells. Results are presented as fold induction over EV (EV = 1). (G) ChIP analysis of FoxO1 binding to the Ocn promoter and the first intron in primary osteoblasts. (H) Changes in uncarboxylated or undercarboxylated Ocn in serum of WT and Foxo1ob–/–Esp+/– mice; n = 5 mice/group. Values are presented as percent of total osteocalcin present in the serum. In all panels, data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test for A–C, F, and H and ANOVA for D and E. Mice were 2–3 months of age.
Figure 6. Esp mediates the effect of Foxo1 on osteocalcin carboxylation and glucose homeostasis.
(A) Real-time PCR analysis of Esp expression in femurs of WT and Foxo1ob–/– bones; n = 3 mice/group. (B) GTT in WT and Foxo1ob+/–Esp+/– mice; n = 5 mice/group. (C) ITT in WT and Foxo1ob+/–Esp+/– mice; n = 5 mice/group. (D) GSIS in WT and Foxo1ob+/–Esp+/– mice; n = 4 mice/group. (E) Cotransfection analysis of FoxO1 interaction with the Esp promoter in Cos-7 cells. Results are presented as fold induction over EV (EV = 1). (F) ChIP analysis of FoxO1 binding to the Esp promoter in primary osteoblasts. In all panels, data are presented as mean ± SEM. Lanes were run on the same gel but were not contiguous. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test for A and E and ANOVA for B–D. Mice were 2 months of age.
Figure 7. Foxo1 deletion in osteoblasts protects from HFD-induced obesity.
(A and B) Body weight curve (A) and MRI analysis of fat content (B) of WT and Foxo1ob–/– mice on normal or high-fat diet; n = 5 mice/group. Basal is considered 1 and is the fat content of each experimental group at 0 weeks, i.e., before the onset of HFD. (C) Perigonadal fat pad weight in WT and Foxo1ob–/– mice on normal or high-fat diet; n = 5 mice/group. (D and E) ITT (D) and GTT (E) analysis in WT and Foxo1ob–/– mice on normal or high-fat diet; n = 5 mice/group. CD, normal control diet. In all panels, data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s t test. In C, letters above the bars denote significant differences, where b is different from a; c is different from a and b; and a,b is different from c. In A–C, mice were 1 month old when placed on HFD. In D and E, mice were 3 months of age.
Comment in
- The FOX(O1) blasts off.
Novack DV. Novack DV. Cell Metab. 2010 Mar 3;11(3):175-6. doi: 10.1016/j.cmet.2010.02.009. Cell Metab. 2010. PMID: 20197048
Similar articles
- FoxO1 protein cooperates with ATF4 protein in osteoblasts to control glucose homeostasis.
Kode A, Mosialou I, Silva BC, Joshi S, Ferron M, Rached MT, Kousteni S. Kode A, et al. J Biol Chem. 2012 Mar 16;287(12):8757-68. doi: 10.1074/jbc.M111.282897. Epub 2012 Feb 1. J Biol Chem. 2012. PMID: 22298775 Free PMC article. - Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts.
Yang S, Xu H, Yu S, Cao H, Fan J, Ge C, Fransceschi RT, Dong HH, Xiao G. Yang S, et al. J Biol Chem. 2011 May 27;286(21):19149-58. doi: 10.1074/jbc.M110.197905. Epub 2011 Apr 6. J Biol Chem. 2011. PMID: 21471200 Free PMC article. - 1α,25-Dihydroxyvitamin D3 promotes bone formation by promoting nuclear exclusion of the FoxO1 transcription factor in diabetic mice.
Xiong Y, Zhang Y, Xin N, Yuan Y, Zhang Q, Gong P, Wu Y. Xiong Y, et al. J Biol Chem. 2017 Dec 8;292(49):20270-20280. doi: 10.1074/jbc.M117.796367. Epub 2017 Oct 17. J Biol Chem. 2017. PMID: 29042442 Free PMC article. - [Pivotal role of skeletal tissues in the regulation mechanisms for physiological functions mediated by multiple organ networks].
Hinoi E. Hinoi E. Yakugaku Zasshi. 2012;132(6):721-5. doi: 10.1248/yakushi.132.721. Yakugaku Zasshi. 2012. PMID: 22687731 Review. Japanese. - [Bone remodeling and glucose/lipid metabolism].
Yoshizawa T. Yoshizawa T. Clin Calcium. 2011 May;21(5):709-14. Clin Calcium. 2011. PMID: 21532121 Review. Japanese.
Cited by
- Diabetes, diabetic complications, and fracture risk.
Oei L, Rivadeneira F, Zillikens MC, Oei EH. Oei L, et al. Curr Osteoporos Rep. 2015 Apr;13(2):106-15. doi: 10.1007/s11914-015-0260-5. Curr Osteoporos Rep. 2015. PMID: 25648962 Free PMC article. Review. - The class II histone deacetylase HDAC4 regulates cognitive, metabolic and endocrine functions through its expression in osteoblasts.
Makinistoglu MP, Karsenty G. Makinistoglu MP, et al. Mol Metab. 2014 Nov 1;4(1):64-9. doi: 10.1016/j.molmet.2014.10.004. eCollection 2015 Jan. Mol Metab. 2014. PMID: 25685691 Free PMC article. - The effects of muscle contraction and recombinant osteocalcin on insulin sensitivity ex vivo.
Levinger I, Lin X, Zhang X, Brennan-Speranza TC, Volpato B, Hayes A, Jerums G, Seeman E, McConell G. Levinger I, et al. Osteoporos Int. 2016 Feb;27(2):653-63. doi: 10.1007/s00198-015-3273-0. Epub 2015 Aug 11. Osteoporos Int. 2016. PMID: 26259649 - FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin.
Kode A, Mosialou I, Silva BC, Rached MT, Zhou B, Wang J, Townes TM, Hen R, DePinho RA, Guo XE, Kousteni S. Kode A, et al. J Clin Invest. 2012 Oct;122(10):3490-503. doi: 10.1172/JCI64906. Epub 2012 Sep 4. J Clin Invest. 2012. PMID: 22945629 Free PMC article. - Therapeutic potentials and modulatory mechanisms of fatty acids in bone.
Bao M, Zhang K, Wei Y, Hua W, Gao Y, Li X, Ye L. Bao M, et al. Cell Prolif. 2020 Feb;53(2):e12735. doi: 10.1111/cpr.12735. Epub 2019 Dec 4. Cell Prolif. 2020. PMID: 31797479 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK078042/DK/NIDDK NIH HHS/United States
- R01 AR045548/AR/NIAMS NIH HHS/United States
- R01 AR055931/AR/NIAMS NIH HHS/United States
- AR055931/AR/NIAMS NIH HHS/United States
- R01 AR054447/AR/NIAMS NIH HHS/United States
- P30 DK063608/DK/NIDDK NIH HHS/United States
- AR054447/AR/NIAMS NIH HHS/United States
- R56 AR054447/AR/NIAMS NIH HHS/United States
- DK063608-07/DK/NIDDK NIH HHS/United States
- DK078042/DK/NIDDK NIH HHS/United States
- AR045548/AR/NIAMS NIH HHS/United States
- R01 DK080756/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous