Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration - PubMed (original) (raw)
. 2010 May;24(5):1376-90.
doi: 10.1096/fj.09-146308. Epub 2009 Dec 29.
Michael S Lustgarten, Yuhong Liu, Florian L Muller, Arunabh Bhattacharya, Hanyu Liang, Adam B Salmon, Susan V Brooks, Lisa Larkin, Christopher R Hayworth, Arlan Richardson, Holly Van Remmen
Affiliations
- PMID: 20040516
- PMCID: PMC2987499
- DOI: 10.1096/fj.09-146308
Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration
Youngmok C Jang et al. FASEB J. 2010 May.
Abstract
Oxidative stress has been implicated in the etiology of age-related muscle loss (sarcopenia). However, the underlying mechanisms by which oxidative stress contributes to sarcopenia have not been thoroughly investigated. To directly examine the role of chronic oxidative stress in vivo, we used a mouse model that lacks the antioxidant enzyme CuZnSOD (Sod1). Sod1(-/-) mice are characterized by high levels of oxidative damage and an acceleration of sarcopenia. In the present study, we demonstrate that muscle atrophy in Sod1(-/-) mice is accompanied by a progressive decline in mitochondrial bioenergetic function and an elevation of mitochondrial generation of reactive oxygen species. In addition, Sod1(-/-) muscle exhibits a more rapid induction of mitochondrial-mediated apoptosis and loss of myonuclei. Furthermore, aged Sod1(-/-) mice show a striking increase in muscle mitochondrial content near the neuromuscular junctions (NMJs). Despite the increase in content, the function of mitochondria is significantly impaired, with increased denervated NMJs and fragmentation of acetylcholine receptors. As a consequence, contractile force in aged Sod1(-/-) muscles is greatly diminished. Collectively, we show that Sod1(-/-) mice display characteristics of normal aging muscle in an accelerated manner and propose that the superoxide-induced NMJ degeneration and mitochondrial dysfunction are potential mechanisms of sarcopenia.
Figures
Figure 1.
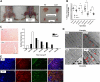
Age-dependent loss of muscle mass corresponds to increased mitochondria content and oxidative fibers in _Sod1_−/− mice. A) Left panel: gross morphology of skinned hindlimb muscles of 20-mo-old _Sod1_−/− and WT mice. Right panel: comparison of individual muscles. B) Comparison of age-associated changes in wet weight of gastrocnemius mass normalized to body weight. C) Top panels: gastrocnemius cross section at 20 mo of age from hematoxylin and eosin stain. Bottom panel: frequency distribution of fiber cross-sectional area (μm2) of gastrocnemius muscle in WT and _Sod1_−/− at 20 mo (_n_=4). D) EM images of gastrocnemius at 14 mo (top panels) and at 22 mo (bottom panels). Arrows and arrowheads indicate increased mitochondria. E) Immunofluorescence images of muscle fiber types from WT (a, c, e, g) and _Sod1_−/− (b, d, f, h) gastrocnemius muscle at 20 mo. a, b) Type IIb (blue). c, d) Type IIa (red). e, f) Type I (green). g, h) Merged images. Scale bars = 50 μm (C); 2 μm (D); 100 μm (E).
Figure 2.
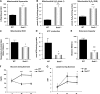
Mitochondrial dysfunction and increased ROS generation in aged _Sod1_−/− mice. A) Extramitochondrial superoxide generation in isolated mitochondria, measured using EPR. Succinate was used as substrate. B) Rate of mitochondrial H2O2 production measured using amplex red in state 1 (left panel) and in response to complex I-linked substrate, glutamate, and malate (right panel). C) Mitochondrial oxygen consumption, expressed as RCR. D) Rate of ATP generation in isolated mitochondria at 20 mo (_n_=6). E) Treadmill endurance test (run to exhaustion) results from 12- to 14-mo-old WT and _Sod1_−/− mice. (_n_=8–10) F, G) Plasma glucose (F) and plasma lactate (G) levels measured immediately following exercise. Values are means ±
se
. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 3.
Elevation of mitochondrial-mediated apoptosis in aged _Sod1_−/− mice. A) Left panel: induction of mitochondrial permeability transition pore (MPTP) opening, measured by a decrease in light absorbance at 540 nm. WT and _Sod1_−/− muscle mitochondria were treated with 200 μM Ca2+. Right panel: quantification of _V_max from the MPTP experiment (_n_=6). B) Left panels: Ca2+-induced cytochrome c and AIF release, measured by Western blot analyses. S, supernatant (released from mitochondria); P, pellet (intact). Right panels: quantification of cytochrome c (top) and AIF release (bottom) in supernatant (_n_=6). Fold change normalized to WT control value. C) Up-regulation of proapoptotic proteins, Bak and Bax, and down-regulation of antiapoptotic proteins, Bcl-2 and Bcl-XL, measured by Western blot analyses. Actin was used as a loading control for each protein; one representative blot is shown.
Figure 4.
Elevation of caspase-3 activity and apoptosis in aged _Sod1_−/− mice. A) Left panels: cell-free apoptosis, measured by liver nuclei treated with buffer alone (top left), WT muscle cytosolic fraction alone (top right), WT mitochondria and WT cytosol (bottom left), and _Sod1_−/− mitochondria and _Sod1_−/− cytosol (bottom right). Arrowhead indicates nuclear blebbing. Right panel: quantification of apoptotic nuclei, measured 4 h after incubation. Assay was run in duplicates and repeated with mitochondria isolated from different animals (_n_=4). B) Caspase-3 activity, measured by cleavage of synthetic peptide _N-_acetyl-DEVD-AMC (_n_=6). C) Apoptosis, determined by quantification of DNA fragmentation (mononucleosome and oligonucleosome) (_n_=6). Values are means ±
se
. *P < 0.05; **P < 0.01_. D_) EM images of myonuclei undergoing apoptotic changes. Arrows indicate nuclear membrane invagination; arrowhead indicates chromatin condensation. Scale bars = 2 μm.
Figure 5.
Aged _Sod1_−/− mice exhibit decreases in myonuclei number and fiber diameter. A) Single-fiber size comparison of WT and _Sod1_−/− gastrocnemius muscle at 20 mo. Arrows indicate areas lacking myonuclei. B) Quantification of myonuclei per 500 μm in both WT and _Sod1_−/− gastrocnemius muscle at 20 mo. C) Quantification of average fiber diameter across single fibers in gastrocnemius muscle from WT and _Sod1_−/− mice at 20 mo. D) Quantification of myonuclear domain, measured by fiber volume per myonuclei number. E) EM of fibers showing dystrophic/necrotic fiber in _Sod1_−/− muscle at 20 mo. Black arrows indicate abnormal mitochondria; arrowheads indicate lipid vacuoles. Scale bars = 50 μm (A); 2 μm (E). Values are means ±
se
. *P < 0.05; ***P < 0.001.
Figure 6.
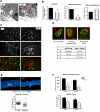
Aged _Sod1_−/− mice exhibit alterations in NMJs and increased denervation. A) EM analysis of NMJ of gastrocnemius at 20 mo. Arrows in WT and arrowheads in _Sod1_−/− indicate synaptic cleft. B) SSM function. Left panel: RCR, measured from SSM from hindlimb muscle at 20 mo. Middle panel: ATP production from SSM, measured at 20 mo. Right panel: rate of H2O2 production from SSM at 20 mo. C) NMJ immunofluorescence images from gastrocnemius at 20 mo. Top panels: morphology of postsynaptic AChRs stained with Alexa 488-conjugated α-bungarotoxin. Middle panels: morphology of presynaptic motor neurons in Thy1 YFP transgenic mice (C57BL/6J background) crossbred to WT and _Sod1_−/− mice. Arrows indicate sprouting and thinning of motor neurons. Bottom panels: overlay images of AChR and motor neuron images shown in top panels. D) Rate of denervation score assessment (top panels) and quantification of denervation score at ∼18 mo (bottom panel). E) Comparison of NMJs in single fibers (top panels) and correlation of fiber diameter and NMJ fragmentation (bottom panel). Arrows indicate NMJs in WT and _Sod1_−/− fibers. F) In situ isometric contraction properties. Maximum isometric force (top) and specific force (bottom). Values are means ±
se
. *P < 0.05; #P < 0.05; **P < 0.01; ***P < 0.001. Scale bars = 2 μm (A); 50 μm (D)
Figure 7.
Disruption of neuromuscular junction proteins in aged _Sod1_−/− mice. A) Top panels: comparison of _z_-stacked images of Alexa 488-conjugated α-bungarotoxin-stained AChR. Bottom panel: quantification of fragmented AChR at 18 mo. Scale bar = 50 μm. B) AChR-mRNA transcript (right panel) and protein expression (left panel) at 18–20 mo. C) Rapsyn protein expression at 20 mo. D) Calpain protein levels at 18–20 mo. Values are means ±
se
. *P < 0.05; **P < 0.01; ***P < 0.001.
Similar articles
- Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD.
Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL, Chaudhuri A, Qi W, Li Y, Huang JY, Verdin E, Richardson A, Van Remmen H. Jang YC, et al. Aging Cell. 2012 Oct;11(5):770-82. doi: 10.1111/j.1474-9726.2012.00843.x. Epub 2012 Aug 2. Aging Cell. 2012. PMID: 22672615 Free PMC article. - Deletion of Neuronal CuZnSOD Accelerates Age-Associated Muscle Mitochondria and Calcium Handling Dysfunction That Is Independent of Denervation and Precedes Sarcopenia.
Su Y, Claflin DR, Huang M, Davis CS, Macpherson PCD, Richardson A, Van Remmen H, Brooks SV. Su Y, et al. Int J Mol Sci. 2021 Oct 4;22(19):10735. doi: 10.3390/ijms221910735. Int J Mol Sci. 2021. PMID: 34639076 Free PMC article. - Neuron specific reduction in CuZnSOD is not sufficient to initiate a full sarcopenia phenotype.
Sataranatarajan K, Qaisar R, Davis C, Sakellariou GK, Vasilaki A, Zhang Y, Liu Y, Bhaskaran S, McArdle A, Jackson M, Brooks SV, Richardson A, Van Remmen H. Sataranatarajan K, et al. Redox Biol. 2015 Aug;5:140-148. doi: 10.1016/j.redox.2015.04.005. Epub 2015 Apr 15. Redox Biol. 2015. PMID: 25917273 Free PMC article. - Accelerated sarcopenia in Cu/Zn superoxide dismutase knockout mice.
Deepa SS, Van Remmen H, Brooks SV, Faulkner JA, Larkin L, McArdle A, Jackson MJ, Vasilaki A, Richardson A. Deepa SS, et al. Free Radic Biol Med. 2019 Feb 20;132:19-23. doi: 10.1016/j.freeradbiomed.2018.06.032. Epub 2018 Jul 2. Free Radic Biol Med. 2019. PMID: 30670156 Free PMC article. Review. - Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting.
Tintignac LA, Brenner HR, Rüegg MA. Tintignac LA, et al. Physiol Rev. 2015 Jul;95(3):809-52. doi: 10.1152/physrev.00033.2014. Physiol Rev. 2015. PMID: 26109340 Review.
Cited by
- Loss of the antioxidant enzyme CuZnSOD (Sod1) mimics an age-related increase in absolute mitochondrial DNA copy number in the skeletal muscle.
Masser DR, Clark NW, Van Remmen H, Freeman WM. Masser DR, et al. Age (Dordr). 2016 Aug;38(4):323-333. doi: 10.1007/s11357-016-9930-1. Epub 2016 Jul 21. Age (Dordr). 2016. PMID: 27444179 Free PMC article. - Oxidative stress and redox regulation on hippocampal-dependent cognitive functions.
Huang TT, Leu D, Zou Y. Huang TT, et al. Arch Biochem Biophys. 2015 Jun 15;576:2-7. doi: 10.1016/j.abb.2015.03.014. Epub 2015 Mar 20. Arch Biochem Biophys. 2015. PMID: 25797440 Free PMC article. Review. - The Role of Autophagy, Mitophagy and Lysosomal Functions in Modulating Bioenergetics and Survival in the Context of Redox and Proteotoxic Damage: Implications for Neurodegenerative Diseases.
Redmann M, Darley-Usmar V, Zhang J. Redmann M, et al. Aging Dis. 2016 Mar 15;7(2):150-62. doi: 10.14336/AD.2015.0820. eCollection 2016 Mar. Aging Dis. 2016. PMID: 27114848 Free PMC article. Review. - Appendicular skeletal muscle mass: A more sensitive biomarker of disease severity than BMI in adults with mitochondrial diseases.
Hou Y, Xie Z, Zhao X, Yuan Y, Dou P, Wang Z. Hou Y, et al. PLoS One. 2019 Jul 25;14(7):e0219628. doi: 10.1371/journal.pone.0219628. eCollection 2019. PLoS One. 2019. PMID: 31344055 Free PMC article. - Cross talk between SOD1 and the mitochondrial UPR in cancer and neurodegeneration.
Gomez M, Germain D. Gomez M, et al. Mol Cell Neurosci. 2019 Jul;98:12-18. doi: 10.1016/j.mcn.2019.04.003. Epub 2019 Apr 24. Mol Cell Neurosci. 2019. PMID: 31028834 Free PMC article. Review.
References
- Mansouri A, Muller F L, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang T T, Epstein C J, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127:298–306. - PubMed
- Moylan J S, Reid M B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. - PubMed
- Lexell J, Taylor C C, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous