The gut microbiota modulates host energy and lipid metabolism in mice - PubMed (original) (raw)
The gut microbiota modulates host energy and lipid metabolism in mice
Vidya R Velagapudi et al. J Lipid Res. 2010 May.
Abstract
The gut microbiota has recently been identified as an environmental factor that may promote metabolic diseases. To investigate the effect of gut microbiota on host energy and lipid metabolism, we compared the serum metabolome and the lipidomes of serum, adipose tissue, and liver of conventionally raised (CONV-R) and germ-free mice. The serum metabolome of CONV-R mice was characterized by increased levels of energy metabolites, e.g., pyruvic acid, citric acid, fumaric acid, and malic acid, while levels of cholesterol and fatty acids were reduced. We also showed that the microbiota modified a number of lipid species in the serum, adipose tissue, and liver, with its greatest effect on triglyceride and phosphatidylcholine species. Triglyceride levels were lower in serum but higher in adipose tissue and liver of CONV-R mice, consistent with increased lipid clearance. Our findings show that the gut microbiota affects both host energy and lipid metabolism and highlights its role in the development of metabolic diseases.
Figures
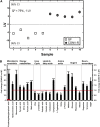
Fig. 1.
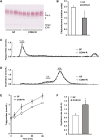
Serum metabolite profiles in CONV-R compared with GF mice. A: PLS/DA of serum metabolites from GF (n = 5) and CONV-R (n = 5) mice after a 4 h fast. Scores for latent variable LV1 and sample are depicted. Regression coefficients and VIP scores for the top ranked metabolites are listed in supplementary Table I. B: Selected serum metabolites that are significantly different in CONV-R (n = 5) compared with GF (n = 5) mice. For a complete list of microbially altered serum metabolites, see supplementary Table II. Data are expressed as mean values ± SEM. * P < 0.05; ** P < 0.01; and *** P < 0.001 compared with GF mice.
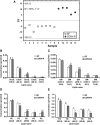
Fig. 2.
Serum lipidomic profiles in CONV-R compared with GF mice. A: PLS/DA of serum lipids from GF (n = 8) and CONV-R (n = 5) mice after a 4 h fast. Scores for latent variable LV1 and sample are depicted. Regression coefficients and VIP scores for the top ranked lipids are listed in supplementary Table III. B-E: Absolute concentrations of the most abundant cholesteryl esters (ChoE), sphingomyelins (SM), phosphatidylcholines (PC), and triglycerides (TG) in serum from GF and CONV-R mice. For a complete list of microbially altered serum lipids, see supplementary Table IV. Data are expressed as mean values ± SEM. * P < 0.05; ** P < 0.01; and *** P < 0.001 compared with GF mice.
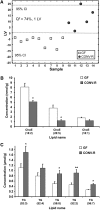
Fig. 3.
Lipidomic profiles of epidiymal adipose tissue in CONV-R compared with GF mice. A: PLS/DA of adipose lipids from GF (n = 9) and CONV-R (n = 5) mice after a 4 h fast. Scores for latent variable LV1 and sample are depicted. Regression coefficients and VIP scores for the top ranked lipids are listed in supplementary Table V. B–C: Absolute concentrations of the most abundant phosphatidylcholines (PC) and triglycerides (TG) in adipose tissue from GF and CONV-R mice. For a complete list of microbially altered adipose lipids, see supplementary Table VI. Data are expressed as mean values ± SEM. * P < 0.05 compared with GF mice.
Fig. 4.
Lipidomic profiles of liver tissue in CONV-R compared with GF mice. A: PLS/DA of liver lipids from GF (n = 9) and CONV-R (n = 5) mice after a 4 h fast. Scores for latent variable LV1 and sample are depicted. Regression coefficients and VIP scores for the top ranked lipids are listed in supplementary Table VII. B–C: Absolute concentrations of the most abundant cholesteryl esters (ChoE) and triglycerides (TG) in liver tissue from GF and CONV-R mice. For a complete list of microbially altered liver lipids, see supplementary Table VIII. Data are expressed as mean values ± SEM. * P < 0.05; ** P < 0.01 compared with GF mice.
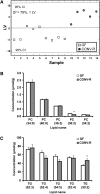
Fig. 5.
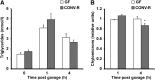
Serum lipoprotein profiles in CONV-R compared with GF mice. A: Representative agarose gel of the serum samples from GF (n = 4) and CONV-R (n = 5) mice where chylomicrons are at the origin, VLDLs in the preβ band, LDL in the β band, and HDL in the α band. B: Quantification of the agarose gels in (A). C: Total triglycerides measured in gel chromatography fractions of pooled serum (n = 5 per group) from GF and CONV-R mice after a 4 h fast. Fractions containing VLDL are indicated. D: Total cholesterol measured in the same gel chromatography fractions as in C. Fractions containing LDL and HDL are indicated. E: Triglycerides in serum from GF (n = 6) and CONV-R (n = 5) mice after an overnight fast and administration of Triton WR 1339. F: VLDL production rates based on data in E. Data are expressed as mean values ± SEM. * P < 0.05 compared with GF mice.
Fig. 6.
Postprandial lipid uptake in CONV-R and GF mice. A: Serum triglyceride levels in GF (n = 7–8/ time point) and CONV-R (n = 6/time point) before and 1 and 4 h after an intragastric cream (36% fat) bolus. B: Levels of chylomicron remnant levels in the same serum as in A analyzed by agarose gel electrophoresis. * P < 0.05 compared with GF mice.
Fig. 7.
Summary of the microbiota-induced changes in energy and lipid metabolism identified in our study. Energy metabolites are increased in the serum and are reflected by an upregulation of related genes in the liver transcriptome. Triglyceride (TG) levels are decreased in the serum and increased in adipose tissue and the liver of CONV-R mice after a 4 h fast. The serum reduction in TG is caused by a decrease in chylomicrons (CM). Lipid absorption is not reduced in CONV-R mice, and we propose that lipid clearance is increased. In agreement, we have previously shown that a microbiota increases suppression of angiopoietin-like protein 4/fasting-induced adipose factor (Angptl4/Fiaf) (16), an inhibitor of LPL, which will promote increased lipid clearance. VLDL production in the liver is increased in CONV-R mice, which may explain why VLDL-TG serum levels are not reduced in CONV-R mice despite increased clearance. Furthermore, phosphatidylcholine (16:0/18:1), which has been identified as a potent endogenous PPARα ligand, is increased in the serum and liver of CONV-R mice. Increased activation of PPARα may promote increased lipid clearance by inducing expression of LPL.
Similar articles
- Metabolomics characterization of energy metabolism reveals glycogen accumulation in gut-microbiota-lacking mice.
Chuang HL, Huang YT, Chiu CC, Liao CD, Hsu FL, Huang CC, Hou CC. Chuang HL, et al. J Nutr Biochem. 2012 Jul;23(7):752-8. doi: 10.1016/j.jnutbio.2011.03.019. Epub 2011 Aug 12. J Nutr Biochem. 2012. PMID: 21840188 - Gut microbiota modulate the metabolism of brown adipose tissue in mice.
Mestdagh R, Dumas ME, Rezzi S, Kochhar S, Holmes E, Claus SP, Nicholson JK. Mestdagh R, et al. J Proteome Res. 2012 Feb 3;11(2):620-30. doi: 10.1021/pr200938v. Epub 2011 Nov 21. J Proteome Res. 2012. PMID: 22053906 - Gut microbiota affects lens and retinal lipid composition.
Oresic M, Seppänen-Laakso T, Yetukuri L, Bäckhed F, Hänninen V. Oresic M, et al. Exp Eye Res. 2009 Nov;89(5):604-7. doi: 10.1016/j.exer.2009.06.018. Epub 2009 Jul 8. Exp Eye Res. 2009. PMID: 19591827 - Effects of the gut microbiota on obesity and glucose homeostasis.
Greiner T, Bäckhed F. Greiner T, et al. Trends Endocrinol Metab. 2011 Apr;22(4):117-23. doi: 10.1016/j.tem.2011.01.002. Epub 2011 Feb 23. Trends Endocrinol Metab. 2011. PMID: 21353592 Review. - Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism.
Caesar R, Fåk F, Bäckhed F. Caesar R, et al. J Intern Med. 2010 Oct;268(4):320-8. doi: 10.1111/j.1365-2796.2010.02270.x. J Intern Med. 2010. PMID: 21050286 Review.
Cited by
- Antibiotics Suppress Activation of Intestinal Mucosal Mast Cells and Reduce Dietary Lipid Absorption in Sprague-Dawley Rats.
Sato H, Zhang LS, Martinez K, Chang EB, Yang Q, Wang F, Howles PN, Hokari R, Miura S, Tso P. Sato H, et al. Gastroenterology. 2016 Nov;151(5):923-932. doi: 10.1053/j.gastro.2016.07.009. Epub 2016 Jul 18. Gastroenterology. 2016. PMID: 27436071 Free PMC article. - Serum metabolomics in a Helicobacter hepaticus mouse model of inflammatory bowel disease reveal important changes in the microbiome, serum peptides, and intermediary metabolism.
Lu K, Knutson CG, Wishnok JS, Fox JG, Tannenbaum SR. Lu K, et al. J Proteome Res. 2012 Oct 5;11(10):4916-26. doi: 10.1021/pr300429x. Epub 2012 Sep 27. J Proteome Res. 2012. PMID: 22957933 Free PMC article. - Mechanisms of hepatic and renal injury in lipid metabolism disorders in metabolic syndrome.
Rong J, Zhang Z, Peng X, Li P, Zhao T, Zhong Y. Rong J, et al. Int J Biol Sci. 2024 Sep 9;20(12):4783-4798. doi: 10.7150/ijbs.100394. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309427 Free PMC article. Review. - Functional interactions between the gut microbiota and host metabolism.
Tremaroli V, Bäckhed F. Tremaroli V, et al. Nature. 2012 Sep 13;489(7415):242-9. doi: 10.1038/nature11552. Nature. 2012. PMID: 22972297 Review. - Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-Vibrio symbiosis.
Heath-Heckman EA, Peyer SM, Whistler CA, Apicella MA, Goldman WE, McFall-Ngai MJ. Heath-Heckman EA, et al. mBio. 2013 Apr 2;4(2):e00167-13. doi: 10.1128/mBio.00167-13. mBio. 2013. PMID: 23549919 Free PMC article.
References
- Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. 2006. Microbial ecology: human gut microbes associated with obesity. Nature. 444: 1022–1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources