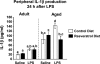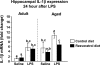Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice - PubMed (original) (raw)
Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice
Jayne Abraham et al. Rejuvenation Res. 2009 Dec.
Abstract
Aged mice treated peripherally with lipopolysaccharide (LPS) show an exaggerated neuroinflammatory response and cognitive deficits compared to adults. Considerable evidence suggests resveratrol, a polyphenol found in red grapes, has potent antiinflammatory effects in the periphery, but its effects on the central inflammatory response and cognitive behavior are unknown. Therefore, the current study investigated if resveratrol dietary supplementation would inhibit neuroinflammation as well as behavioral and cognitive deficits in aged mice given LPS to mimic a peripheral infection. In initial studies, adult (3-6 months) and aged (22-24 months) mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected intraperitoneally (i.p.) with saline or LPS, and locomotor activity and spatial working memory were assessed. As anticipated, deficits in locomotor activity and spatial working memory indicated aged mice are more sensitive to LPS compared to adults. More importantly, the LPS-induced deficits in aged animals were mitigated by dietary supplementation of resveratrol. In addition, resveratrol consumption reduced LPS-induced interleukin-1beta (IL-1beta) in plasma and the IL-1beta mRNA in the hippocampus of aged mice. Finally, pretreatment of BV-2 microglial cells with resveratrol potently inhibited LPS-induced IL-1beta production. These data show that aged mice are more sensitive than adult mice to both the inflammatory and cognitive effects of peripheral immune stimulation and suggest that resveratrol may be useful for attenuating acute cognitive disorders in elderly individuals with an infection.
Figures
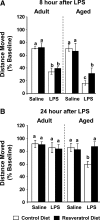
FIG. 1.
Resveratrol protected aged mice but not adult mice from lipopolysaccharide (LPS)-induced deficits in locomotor behavior. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected peripherally with saline or LPS. Locomotor activity was measured for both adult and aged mice at 8 h (A) and 24 h (B) after LPS injection. Bars represent the mean ± standard error of the mean (SEM) (n = 10–11). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
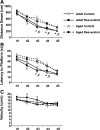
FIG. 2.
Performance of adult and aged mice during a 5-day acquisition phase in the Morris water maze. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks, and during the fourth week of diet supplementation animals were trained in a 5-day acquisition phase. Distance swam (A), latency to platform (B), and swim speed (C) were measured for both adult and aged mice. Data points represent the mean ± standard error of the mean (SEM) (n = 11–13). Means marked with and asterisk (*) or number sign (#) are significantly different (p < 0.05) from aged treatment-matched baseline controls, respectively.
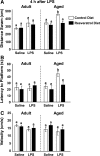
FIG. 3.
Resveratrol improves impaired spatial working memory in aged mice 4 h post LPS injection. After 5 days of acquisition training, mice were evaluated in a reversal test 4 h after lipopolysaccharide (LPS) injection. Distance swam to platform (A), latency to find the platform (B), and swim speed (C) were evaluated for both adult and aged mice. Bars represent the mean ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
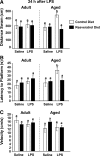
FIG. 4.
Resveratrol improves impaired spatial working memory in aged mice 24 h post LPS injection. After 5 days of acquisition training, mice were evaluated in a reversal test 24 h after lipopolysaccharide (LPS) injection. Distance swam to platform (A), latency to find the platform (B), and swim speed (C) were evaluated for both adult and aged mice. Bars represent the mean ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a or b) are significantly different (p < 0.05) from each other.
FIG. 5.
Dietary supplementation with resveratrol inhibited the LPS-induced increase in interleukin-1β (IL-1β) in periphery of aged mice. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected peripherally with saline or lipopolysaccharide (LPS). After the final behavioral test or cognitive test (24 h after injection), blood was collected and IL-1β production was measured by an IL-1β Quantikine assay. Bars represent means ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
FIG. 6.
Dietary supplementation with resveratrol inhibited the lipopolysaccharide (LPS)-induced increase in interleukin-1β (IL-1β) mRNA in aged mice. Adult and aged mice were provided control or resveratrol-supplemented diet for 4 weeks and then injected peripherally with saline or LPS. After the final behavioral test or cognitive test (24 h after injection), hippocampal tissue was collected and IL-1β mRNA was measured by quantitative real-time PCR. Bars represent means ± standard error of the mean (SEM) (n = 11–13). Means with different letters (a, b, or c) are significantly different (p < 0.05) from each other.
FIG. 7.
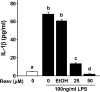
Resveratrol inhibits lipopolysaccharide (LPS)-induced interleukin-1β (IL-1β) secretion in BV-2 cells. BV-2 cells were pretreated with resveratrol (0–50 μM) for 1 h and stimulated with LPS (100 ng/mL) for a 4-h incubation period. IL-1β secretion was measured by an IL-1β Quantikine immunoassay. Concentration of IL-1β in supernatants from BV-2 cells stimulated with LPS was ∼ 70.0 pg/mL. Bars represent the mean ± standard error of the mean (SEM) from three independent experiments. Means with different letters (a, b, c, or d) are significantly different (p < 0.05) from each other. Resv, Resveratrol; EtOH, ethanol.
Similar articles
- Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice.
Jang S, Dilger RN, Johnson RW. Jang S, et al. J Nutr. 2010 Oct;140(10):1892-8. doi: 10.3945/jn.110.123273. Epub 2010 Aug 4. J Nutr. 2010. PMID: 20685893 Free PMC article. - Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia.
Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Henry CJ, et al. J Neuroinflammation. 2008 May 13;5:15. doi: 10.1186/1742-2094-5-15. J Neuroinflammation. 2008. PMID: 18477398 Free PMC article. - Resveratrol--pills to replace a healthy diet?
Chachay VS, Kirkpatrick CM, Hickman IJ, Ferguson M, Prins JB, Martin JH. Chachay VS, et al. Br J Clin Pharmacol. 2011 Jul;72(1):27-38. doi: 10.1111/j.1365-2125.2011.03966.x. Br J Clin Pharmacol. 2011. PMID: 21410504 Free PMC article. Review. - Lifestyle modifications with anti-neuroinflammatory benefits in the aging population.
Muscat SM, Barrientos RM. Muscat SM, et al. Exp Gerontol. 2020 Dec;142:111144. doi: 10.1016/j.exger.2020.111144. Epub 2020 Nov 2. Exp Gerontol. 2020. PMID: 33152515 Free PMC article. Review.
Cited by
- Plant extracts and omega-3 supplementation modulate hippocampal oxylipin profile in response to LPS-induced neuroinflammation.
Martin M, Debenay E, Bardinet J, Peltier A, Pourtau L, Gaudout D, Layé S, Pallet V, Dinel AL, Joffre C. Martin M, et al. Inflamm Res. 2024 Nov;73(11):2023-2042. doi: 10.1007/s00011-024-01947-9. Epub 2024 Sep 28. Inflamm Res. 2024. PMID: 39340661 Free PMC article. - Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria.
Di Salvo E, Gangemi S, Genovese C, Cicero N, Casciaro M. Di Salvo E, et al. Plants (Basel). 2023 Oct 15;12(20):3579. doi: 10.3390/plants12203579. Plants (Basel). 2023. PMID: 37896042 Free PMC article. Review. - Neuroinflammation in the Central Nervous System: Exploring the Evolving Influence of Endocannabinoid System.
Rathod SS, Agrawal YO, Nakhate KT, Meeran MFN, Ojha S, Goyal SN. Rathod SS, et al. Biomedicines. 2023 Sep 26;11(10):2642. doi: 10.3390/biomedicines11102642. Biomedicines. 2023. PMID: 37893016 Free PMC article. Review. - Role of BDNF Signaling in the Neuroprotective and Memory-enhancing Effects of Flavonoids in Alzheimer's Disease.
Amidfar M, Garcez ML, Askari G, Bagherniya M, Khorvash F, Golpour-Hamedani S, de Oliveira J. Amidfar M, et al. CNS Neurol Disord Drug Targets. 2024;23(8):984-995. doi: 10.2174/1871527323666230912090856. CNS Neurol Disord Drug Targets. 2024. PMID: 37702162 Review. - Interplay between Systemic Glycemia and Neuroprotective Activity of Resveratrol in Modulating Astrocyte SIRT1 Response to Neuroinflammation.
Grabowska AD, Wątroba M, Witkowska J, Mikulska A, Sepúlveda N, Szukiewicz D. Grabowska AD, et al. Int J Mol Sci. 2023 Jul 19;24(14):11640. doi: 10.3390/ijms241411640. Int J Mol Sci. 2023. PMID: 37511397 Free PMC article.
References
- Avital A. Goshen I. Kamsler A. Segal M. Iverfeldt K. Richter-Levin G. Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. - PubMed
- Pugh RC. Fleshner M. Watkins LR. Maier SF. Rudy JW. The immune system and memory consolidation: a role for the cytokine IL- 1beta. Neurosci Biobehav Rev. 2001;25:29–41. - PubMed
- Ben Menachem-Zidon O. Goshen I. Kreisel T. Ben Menahem Y. Reinhartz E. Ben Hur T. Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. - PubMed
- Godbout JP. Chen J. Abraham J. Richwine AF. Berg BM. Kelley KW. Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH069148/MH/NIMH NIH HHS/United States
- R01 MH069148/MH/NIMH NIH HHS/United States
- R01 AG016710/AG/NIA NIH HHS/United States
- T32 DK059802-05/DK/NIDDK NIH HHS/United States
- T32 DK059802/DK/NIDDK NIH HHS/United States
- AG16710/AG/NIA NIH HHS/United States
- 5T32 DK59802/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials