Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions - PubMed (original) (raw)
Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions
Isabel L Jackson et al. Radiat Res. 2010 Jan.
Abstract
The mouse has been used extensively to model radiation injury to the lung, a major dose-limiting organ for radiotherapy. Substantial differences in the timing and sensitivity of this tissue between mouse strains have been reported, with some strains, including C57BL/6, being designated as "fibrosis-prone". Pleural effusions have also been reported to be a prominent problem in many mouse strains, but it remains unclear how this affects the lung function and survival of the standard C57BL/6 mouse. The purpose of this investigation was to re-evaluate this strain in comparison with C57L and CBA mice after whole-thorax irradiation at doses ranging from 10 to 15 Gy. Breathing rate measurements, micro-computerized tomography, lung tissue weight, pleural fluid weight and histopathology showed that the most prominent features were an early phase of pneumonitis (C57L and CBA) followed by a late incidence of massive pleural effusions (CBA and C57BL/6). A remarkable difference was seen between the C57 strains: The C57L mice were exquisitely sensitive to early pneumonitis at 3 to 4 months while C57BL/6 mice showed a delayed response, with most mice presenting with large accumulations of pleural fluid at 6 to 9 months. These results therefore caution against the routine use of C57BL/6 mice in radiation lung experiments because pleural effusions are rarely observed in patients as a consequence of radiotherapy. Future experiments designed to investigate genetic determinants of radiation lung damage should focus on the high sensitivity of the C57L strain (in comparison with CBA or C3H mice) and the possibility that they are more susceptible to pulmonary fibrosis as well as pneumonitis.
Figures
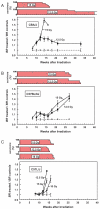
FIG. 1
Breathing rate (BR), expressed as a ratio of controls, and percentage survival plotted as a function of time after single doses of whole-thorax irradiation (200 kVp X rays) to CBA/JIco (panel A), C57BL/6JIco (panel B) and C57L/J (panel C) mice. Each group consisted of five mice at the start. Error bars represent ±1 SEM.
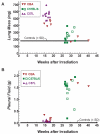
FIG. 2
Fresh lung mass (panel A) and pleural fluid content (panel B) from individual CBA (▼, ▽), C57BL/6 (■, □) and C57L (▲, △) mice. Closed symbols are mice treated at UMCG (CBA/JIco, C57BL/6JIco and C57L/J) with 200 kVp X rays and open symbols are mice (CBA/J, C57BL/6J and C57L/J) treated at MIT with 137Cs γ rays. All animals euthanized before 20 weeks showed severe gross damage (reddened and firm), while 14 of 16 mice euthanized at later times had lungs with moderate to no macroscopic changes but had massive pleural effusions. Shaded area represents the standard deviation (SD) around the mean of five unirradiated C57BL/6JIco control mice. Comparisons of the lung or pleural fluid weights using to the Mann-Whitney U test showed no difference (P > 0.05) between treatments with X rays (UMCG) and γ rays (MIT) for each strain (CBA or C57L mice at 14–18 weeks and C57BL/6 mice at 26–32 weeks).
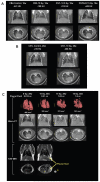
FIG. 3
Panel A: Micro-CT coronal and axial sections through the mid-thorax of control and irradiated mice at 16 weeks after whole-thorax irradiation (137Cs γ rays) to show regional increase in lung parenchyma density (arrows) associated with pneumonitis in treated CBA/J and C57L/J mice but not C57BL/6 mice. Panel B: Micro-CT sections of a control C57L/J mouse and a C57L/J mouse at 28 weeks after 10 Gy whole-thorax irradiation with focal radio-opaque lesions (arrows) indicative of fibrosis. Panel C: Micro-CT images with three-dimensional reconstructions together with coronal and axial T2-weighted MRI of C57BL/6J mice at 28–30 weeks after whole-thorax irradiation showing increased lung density and decreased lung volume associated with the presence of pleural effusions. The circle (*) shows a polypropylene tube containing pleural fluid isolated from another irradiated C57BL/6 mouse.
FIG. 4
Quantitative CT analysis of lung density and volume in individual CBA/J, C57L/J and C57BL/6J mice at 15–16 weeks (closed symbols) or 28–30 weeks (open symbols) after whole-thorax irradiation. * P < 0.05, ** P < 0.01 compared to unirradiated control of the same strain using Mann-Whitney U test. # P < 0.05 for irradiated C57L/J mice compared to CBA/J or C57BL/6J control mice.
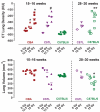
FIG. 5
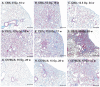
Masson’s trichrome-stained lung sections from different mouse strains. Panel A: Healthy unirradiated control CBA/J mouse with normal tissue architecture. Lung weight = 211 mg, pleural fluid = 0.02 ml. Panel B: 15 Gy-irradiated CBA/J mouse with respiratory distress at 18 weeks showing severe typical inflammatory pneumonitis (alveolitis) affecting most of the lung tissue. Lung weight = 478 mg, pleural fluid = 0.19 ml. Panel C: 12.5 Gy-irradiated CBA/J mouse with respiratory distress at 34 weeks with pleural effusion showing focal subpleural area of foamy macrophages. Lung weight = 242 mg, pleural fluid = 1.68 ml. Panel D: 12.5 Gy-irradiated C57L/J mouse with respiratory distress at 15 weeks with severe pneumonitis. Lung weight = 563 mg, pleural fluid = 0.04 ml. Panel E: 12.5 Gy-irradiated C57L/J mouse at 17 weeks with respiratory distress with focal collagen deposition surrounded by inflammatory cell infiltrate and edema. Lung weight = 589 mg, pleural fluid = 0.04 ml. Panel F: 10 Gy-irradiated C57L/J mouse without respiratory distress at 28 weeks showing focal contracted fibrosis. Lung weight = 303 mg, pleural fluid = 0.05 ml. Panel G: 15 Gy-irradiated C57BL/6J mouse with respiratory distress at 28 weeks with pleural effusion and mostly normal lung appearance. Lung weight = 254 mg, pleural fluid = 1.26 ml. Panel H: 15 Gy-irradiated C57BL/6J mouse with respiratory distress at 28 weeks with pleural effusion showing focal area of subpleural fibrosis. Lung weight = 286 mg, pleural fluid = 1.02 ml. Panel I: 15 Gy-irradiated C57BL/6J mouse with respiratory distress at 32 weeks with moderate effusion and pneumonitis. Lung weight = 375 mg, pleural fluid = 0.57 ml. Bars represent 250 μm.
FIG. 6
The varying development of radiation pneumonitis (Pn) and pleural effusions (Pl) among different rodent strains after thoracic irradiation. The CBA, C57BL/6 and C57L mice from the present studies are compared with CBA/Ca, C57BL/Cbi, their hybrids (CBBF1), WHT, TO and C3H/HeJ mice (12, 13) and August (13) and Brown Norway (BN) (15) rats. The late and intermediate development of pneumonitis in C57BL/Cbi and CBBF1 mice, respectively, was seen only after hemithorax irradiation with shielding of the left lung, which prevented pleural effusions (37). C57L, CBA and C3H mice exhibited an early phase attributed exclusively to direct lung damage (pneumonitis) after whole-thorax irradiation with pathology and timing similar to that seen in patients receiving wide-field radiotherapy (3, 38).
Similar articles
- Gene expression profiles among murine strains segregate with distinct differences in the progression of radiation-induced lung disease.
Jackson IL, Baye F, Goswami CP, Katz BP, Zodda A, Pavlovic R, Gurung G, Winans D, Vujaskovic Z. Jackson IL, et al. Dis Model Mech. 2017 Apr 1;10(4):425-437. doi: 10.1242/dmm.028217. Epub 2017 Jan 26. Dis Model Mech. 2017. PMID: 28130353 Free PMC article. - A survey of changing trends in modelling radiation lung injury in mice: bringing out the good, the bad, and the uncertain.
Dabjan MB, Buck CM, Jackson IL, Vujaskovic Z, Marples B, Down JD. Dabjan MB, et al. Lab Invest. 2016 Sep;96(9):936-49. doi: 10.1038/labinvest.2016.76. Epub 2016 Aug 1. Lab Invest. 2016. PMID: 27479087 Review. - Pulmonary effects of radiation therapy.
Gross NJ. Gross NJ. Ann Intern Med. 1977 Jan;86(1):81-92. doi: 10.7326/0003-4819-86-1-81. Ann Intern Med. 1977. PMID: 319723 Review.
Cited by
- Radiation-induced lung injury is mitigated by blockade of gastrin-releasing peptide.
Zhou S, Nissao E, Jackson IL, Leong W, Dancy L, Cuttitta F, Vujaskovic Z, Sunday ME. Zhou S, et al. Am J Pathol. 2013 Apr;182(4):1248-54. doi: 10.1016/j.ajpath.2012.12.024. Epub 2013 Feb 8. Am J Pathol. 2013. PMID: 23395092 Free PMC article. - Microarray analysis of hub genes, non-coding RNAs and pathways in lung after whole body irradiation in a mouse model.
Aryankalayil MJ, Bylicky MA, Martello S, Chopra S, Sproull M, May JM, Shankardass A, MacMillan L, Vanpouille-Box C, Eke I, Scott KMK, Dalo J, Coleman CN. Aryankalayil MJ, et al. Int J Radiat Biol. 2023;99(11):1702-1715. doi: 10.1080/09553002.2023.2214205. Epub 2023 Jun 1. Int J Radiat Biol. 2023. PMID: 37212632 Free PMC article. - Immune Dysfunction from Radiation Exposure.
Hollingsworth BA, Aldrich JT, Case CM Jr, DiCarlo AL, Hoffman CM, Jakubowski AA, Liu Q, Loelius SG, PrabhuDas M, Winters TA, Cassatt DR. Hollingsworth BA, et al. Radiat Res. 2023 Oct;200(4):396-416. doi: 10.1667/rade-22-00004.1. Epub 2023 Sep 13. Radiat Res. 2023. PMID: 38152282 Free PMC article. - Anti-ceramide Single-Chain Variable Fragment Mitigates Gastrointestinal-Acute Radiation Syndrome and Improves Marrow Reconstitution, Rendering Near-Normal 90-Day Autopsies.
Nagesh PKB, Monette S, Shamu T, Giralt S, Jean SCS, Zhang Z, Fuks Z, Kolesnick R. Nagesh PKB, et al. Int J Radiat Oncol Biol Phys. 2024 Oct 1;120(2):558-569. doi: 10.1016/j.ijrobp.2023.07.038. Epub 2023 Oct 9. Int J Radiat Oncol Biol Phys. 2024. PMID: 37815783 Free PMC article. - Loss of Nrf2 promotes alveolar type 2 cell loss in irradiated, fibrotic lung.
Traver G, Mont S, Gius D, Lawson WE, Ding GX, Sekhar KR, Freeman ML. Traver G, et al. Free Radic Biol Med. 2017 Nov;112:578-586. doi: 10.1016/j.freeradbiomed.2017.08.026. Epub 2017 Sep 1. Free Radic Biol Med. 2017. PMID: 28870520 Free PMC article.
References
- Gross NJ. Pulmonary effects of radiation therapy. Ann. Intern. Med. 1977;86:81–92. - PubMed
- Salazar OM, Rubin P, Keller B, Scarantino C. Systemic (half-body) radiation therapy: response and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 1978;4:937–950. - PubMed
- Van Dyk J, Keane TJ, Kan S, Rider WD, Fryer CJ. Radiation pneumonitis following large single dose irradiation: a re-evaluation based on absolute dose to lung. Int. J. Radiat. Oncol. Biol. Phys. 1981;7:461–467. - PubMed
- Keane TJ, Van Dyk J, Rider WD. Idiopathic interstitial pneumonia following bone marrow transplantation: the relationship with total body irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1981;7:1365–1370. - PubMed
- Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:876–884. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA098452/CA/NCI NIH HHS/United States
- U19 AI067798/AI/NIAID NIH HHS/United States
- 5U19AI067798/AI/NIAID NIH HHS/United States
- 5R01CA098452-04/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources