Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping - PubMed (original) (raw)
Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping
Saji George et al. ACS Nano. 2010.
Abstract
The establishment of verifiably safe nanotechnology requires the development of assessment tools to identify hazardous nanomaterial properties that could be modified to improve nanomaterial safety. While there is a lot of debate of what constitutes appropriate safety screening methods, one approach is to use the assessment of cellular injury pathways to collect knowledge about hazardous material properties that could lead to harm to humans and the environment. We demonstrate the use of a multiparameter cytotoxicity assay that evaluates toxic oxidative stress to compare the effects of titanium dioxide (TiO(2)), cerium oxide (CeO(2)), and zinc oxide (ZnO) nanoparticles in bronchial epithelial and macrophage cell lines. The nanoparticles were chosen on the basis of their volume of production and likelihood of spread to the environment. Among the materials, dissolution of ZnO nanoparticles and Zn(2+) release were capable of ROS generation and activation of an integrated cytotoxic pathway that includes intracellular calcium flux, mitochondrial depolarization, and plasma membrane leakage. These responses were chosen on the basis of the compatibility of the fluorescent dyes that contemporaneously assess their response characteristics by a semiautomated epifluorescence procedure. Purposeful reduction of ZnO cytotoxicity was achieved by iron doping, which changed the material matrix to slow Zn(2+) release. In summary, we demonstrate the utility of a rapid throughput, integrated biological oxidative stress response pathway to perform hazard ranking of a small batch of metal oxide nanoparticles, in addition to showing how this assay can be used to improve nanosafety by decreasing ZnO dissolution through Fe doping.
Figures
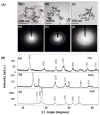
Figure 1. Physicochemical characterisation of metal oxide nanoparticles (NPs)
(A). TEM characterization of the NPs used in this study. (a) TiO2 (b) CeO2 (c) ZnO and SAED of (d) TiO2 (e) CeO2 (f) ZnO NPs. All NPs were prepared and applied to grids as described in the synthesis and experimental section. (B). XRD patterns of TiO2, CeO2, and ZnO (metal oxide) NPs.
Figure 2. Fluorescence probes-properties and utilities
(A) Cytotoxicity markers and probes used in the study. (B) (i) BEAS-2B cells subjected to three hours treatment with different NPs (50 μg/mL) were stained with Hoechst 33342 and JC1, simultaneously. The images of cells were captured by epifluorescence microscopy. Healthy cells (negative control) showed blue nuclei and red cytoplasm indicating healthy mitochondria. Mitochondria with decreased membrane potential cause the monomer formation of JC1 molecules, yielding green fluorescence. The percentage of cells with mitochondrial perturbation was highest in ZnO treatment group. TiO2 treatment also resulted in a small population of cells with unhealthy mitochondria, while CeO2 showed no sign of mitochondrial toxicity. (B) (ii) BEAS-2B cells subjected to three hours treatment with different NPs (50 μg/mL) were stained with Hoechst 33342, Fluo-4 and propidium iodide (PI), simultaneously. Healthy cells showed blue nuclei while cells with membrane damage showed red nuclei. The increase in intracellular Ca2+ is shown as intense green cytoplasm. ZnO treated cells showed a distinct population of cells positive for both PI uptake and increased Fluo-4 intensity. TiO2 and CeO2 showed no significant increase in population of cells positive for PI and Fluo-4.
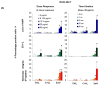
Figure 3. Multi-parametric assay to compare the cytotoxicity of metal oxide NPs in RAW 264.7 and BEAS-2B cells
Cellular plating, particle dispersion and addition to the tissue culture plates, cellular staining and epifluorescence microscopy were performed as described in materials and methods. After exposure to NPs suspended in respective growth medium at 6.125 μg/mL-50 μg/mL for 3 hours (dose response) or exposed to 0,1–6 hours at 50 μg/mL concentration (time kinetics), RAW 264.7 (A) and BEAS-2B cells (B) were stained for 30 minutes with the dye cocktails that contemporaneously assess mitochondrial membrane potential (JC1-1μM), intracellular calcium flux (Fluo4-5μM) and membrane damage (PI- 5μM). The percentage of cells positive for the given cytotoxic response was scored from fluorescence image cells using MetaXpress software. The error bar indicates standard deviation from mean value obtained from nine replicates *p<0.05- comparison is with 0 dose or duration of exposure. Number of repeat experiments = 3
Figure 3. Multi-parametric assay to compare the cytotoxicity of metal oxide NPs in RAW 264.7 and BEAS-2B cells
Cellular plating, particle dispersion and addition to the tissue culture plates, cellular staining and epifluorescence microscopy were performed as described in materials and methods. After exposure to NPs suspended in respective growth medium at 6.125 μg/mL-50 μg/mL for 3 hours (dose response) or exposed to 0,1–6 hours at 50 μg/mL concentration (time kinetics), RAW 264.7 (A) and BEAS-2B cells (B) were stained for 30 minutes with the dye cocktails that contemporaneously assess mitochondrial membrane potential (JC1-1μM), intracellular calcium flux (Fluo4-5μM) and membrane damage (PI- 5μM). The percentage of cells positive for the given cytotoxic response was scored from fluorescence image cells using MetaXpress software. The error bar indicates standard deviation from mean value obtained from nine replicates *p<0.05- comparison is with 0 dose or duration of exposure. Number of repeat experiments = 3
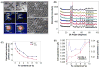
Figure 4. Physicochemical characterisation of iron-doped ZnO NPs
(A) Low resolution TEM of pure ZnO (a) and 10.2 % Fe doped ZnO (b). SAED patterns of pure ZnO (c) and 10.2 % Fe doped ZnO (d). Power spectrum of single crystalline pure (e) and 10.2 % Fe doped ZnO nanoparticles (f) showing no changes in the crystal structures of the NPs. High resolution TEM images of the pure ZnO (g) and 10.2 % Fe doped ZnO NPs (h). (B) XRD patterns of pure and Fe doped ZnO NPs. (C) Equivalent primary particle size obtained using XRD and BET surface area measurements. Error bar indicates standard deviation from mean value. (D) Variation in the lattice parameters with increasing Fe loading. The trend in the change of the parameters are the same for _a_=b and c with increasing Fe content. Error bar indicates standard deviation from mean value.
Figure 5. Distribution of Fe in crystal structure of ZnO NPs
(A) EELS spectrum of 10.2 % Fe doped ZnO NPs. The energy loss value of 708 eV from the L shell (1s2 2p6) shows clear indication of the homogenous Fe distribution in the ZnO matrix. (B) The elemental mapping (EFTEM) of 10% Fe doped ZnO NP (a, b) pre-edge and post-edge of Fe mapping (c) Fe mapping in the wide spot of the NP distribution (d, e) pre-edge and post-edge of Zn (f) Zn mapping in the same wide spot of the NP distribution
Figure 6. Dissolution kinetics and physicochemical characteristics of iron doped ZnO NPs in aqueous media
(A) The rate of zinc release into aqueous solution at pH 7 by the dissolution of pure and Fe-doped ZnO nanoparticles. ZnO dissolution and Zn2+ release was studied by adding 6.7 – 6.9 mg of material to 25 mL of a 0.1 M sodium perchlorate solution at pH 7. The kinetics of Zn 2+ was followed by measuring the amount of nitric acid added to maintain the pH and by periodic sampling to assay for Zn content using ICP-MS. (B) The effect of iron doping on ZnO size and zeta potential. Please notice that the increase in mean particle size with incremental atomic percentages of Fe is due to particle agglomeration, which tended to be larger in the low protein (BEGM) compared to the higher protein (CDMEM) medium.
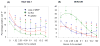
Figure 7. Multi-parametric assay showing the effect of iron doping on the cytotoxicity of nano ZnO
After exposure to NPs suspended in respective growth medium at 6.125 μg/mL-50 μg/mL for 3 hours, cells were stained with our dye cocktail (refer Fig. 3 legend). The percentage of cells positive for a given response parameter was scored using MetaXpress software. (A) RAW 264.7 cells subjected to NP treatment showed a significant decrease in cytotoxic effects such as drop in MMP, increase in intra-cellular calcium level and cell membrane damage as the atomic percentage of Fe in ZnO increases. (B) BEAS-2B cells subjected to nanoparticle treatment showed a significant decrease in cytotoxic effects such as rise in [Ca2+]i and cell membrane damage as the atomic percentage of Fe in ZnO increases. (Error bar indicates standard deviation from mean value obtained from nine replicates. *p<0.05- comparison is with no doping).
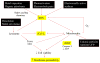
Figure 8. Schematic to illustrate the interrelatedness of cellular responses in our multi-parametric assay and the linkage to nanoparticle-induced ROS production
Nanomaterials induce ROS production as a direct consequence of specific material properties or as a consequence of triggering cellular injury responses leading to oxidant radical generation. ROS production could trigger a range of oxidative stress effects as outlined in the hierarchical oxidative stress model shown in Fig. S1. The induction of cellular toxicity at the highest level of oxidative stress involves a number of interrelated cellular responses that include intracellular Ca2+ release and mitochondrial perturbation leading to cell death with accompanying changes in cell membrane integrity and nuclear PI uptake. We have previously demonstrated that ZnO nanoparticles engage this toxic oxidative stress pathway through an event sequence that includes particle dissolution, shedding of Zn2+, [Ca2+]i flux, superoxide generation and mitochondrial perturbation . The schematic outlines the particle characteristics that could culminate in cytotoxicity.
Similar articles
- Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties.
Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Xia T, et al. ACS Nano. 2008 Oct 28;2(10):2121-34. doi: 10.1021/nn800511k. ACS Nano. 2008. PMID: 19206459 Free PMC article. - Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos.
Xia T, Zhao Y, Sager T, George S, Pokhrel S, Li N, Schoenfeld D, Meng H, Lin S, Wang X, Wang M, Ji Z, Zink JI, Mädler L, Castranova V, Lin S, Nel AE. Xia T, et al. ACS Nano. 2011 Feb 22;5(2):1223-35. doi: 10.1021/nn1028482. Epub 2011 Jan 20. ACS Nano. 2011. PMID: 21250651 Free PMC article. - The fate of ZnO nanoparticles administered to human bronchial epithelial cells.
Gilbert B, Fakra SC, Xia T, Pokhrel S, Mädler L, Nel AE. Gilbert B, et al. ACS Nano. 2012 Jun 26;6(6):4921-30. doi: 10.1021/nn300425a. Epub 2012 Jun 7. ACS Nano. 2012. PMID: 22646753 Free PMC article. - Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism.
Król A, Pomastowski P, Rafińska K, Railean-Plugaru V, Buszewski B. Król A, et al. Adv Colloid Interface Sci. 2017 Nov;249:37-52. doi: 10.1016/j.cis.2017.07.033. Epub 2017 Aug 26. Adv Colloid Interface Sci. 2017. PMID: 28923702 Review. - The toxicology of ion-shedding zinc oxide nanoparticles.
Liu J, Feng X, Wei L, Chen L, Song B, Shao L. Liu J, et al. Crit Rev Toxicol. 2016;46(4):348-84. doi: 10.3109/10408444.2015.1137864. Epub 2016 Feb 25. Crit Rev Toxicol. 2016. PMID: 26963861 Review.
Cited by
- Silver nanoparticle induced immunogenic cell death can improve immunotherapy.
Sargsian A, Koutsoumpou X, Girmatsion H, Egil Ç, Buttiens K, Luci CR, Soenen SJ, Manshian BB. Sargsian A, et al. J Nanobiotechnology. 2024 Nov 10;22(1):691. doi: 10.1186/s12951-024-02951-1. J Nanobiotechnology. 2024. PMID: 39523339 Free PMC article. - Chemical screens for particle-induced macrophage death identifies kinase inhibitors of phagocytosis as targets for toxicity.
Tran UT, Kitami T. Tran UT, et al. J Nanobiotechnology. 2024 Oct 14;22(1):621. doi: 10.1186/s12951-024-02885-8. J Nanobiotechnology. 2024. PMID: 39396993 Free PMC article. - Long-Term Exposure of Fresh and Aged Nano Zinc Oxide Promotes Hepatocellular Carcinoma Malignancy by Up-Regulating Claudin-2.
Yu N, Su M, Wang J, Liu Y, Yang J, Zhang J, Wang M. Yu N, et al. Int J Nanomedicine. 2024 Oct 1;19:9989-10008. doi: 10.2147/IJN.S478279. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39371475 Free PMC article. - Novel composite of nano zinc oxide and nano propolis as antibiotic for antibiotic-resistant bacteria: a promising approach.
Salama SA, Essam D, Tagyan AI, Farghali AA, Khalil EM, Abdelaleim YF, Hozzein WN, Mubarak M, Nasr FA, Eweis AA, Al-Zharani M, Mahmoud R. Salama SA, et al. Sci Rep. 2024 Sep 8;14(1):20894. doi: 10.1038/s41598-024-70490-8. Sci Rep. 2024. PMID: 39245771 Free PMC article. - Introducing the antibacterial and photocatalytic degradation potentials of biosynthesized chitosan, chitosan-ZnO, and chitosan-ZnO/PVP nanoparticles.
Aouadi A, Hamada Saud D, Rebiai A, Achouri A, Benabdesselam S, Mohamed Abd El-Mordy F, Pohl P, Ahmad SF, Attia SM, Abulkhair HS, Ararem A, Messaoudi M. Aouadi A, et al. Sci Rep. 2024 Jun 26;14(1):14753. doi: 10.1038/s41598-024-65579-z. Sci Rep. 2024. PMID: 38926522 Free PMC article.
References
- Nel A, Xia T, Mädler L, Li N. Toxic Potential of Materials at the Nanolevel. Science. 2006;311:622–627. - PubMed
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, et al. Safe Handling of Nanotechnology. Nature. 2006;444:267–269. - PubMed
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding Biophysicochemical Interactions at the Nano-bio Interface. Nat Mater. 2009;8:543–557. - PubMed
- Stasko NA, Johnson CB, Schoenfisch MH, Johnson TA, Holmuhamedov EL. Cytotoxicity of Polypropylenimine Dendrimer Conjugates on Cultured Endothelial Cells. Biomacromolecules. 2007;8:3853–3859. - PubMed
- Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB, et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004;4:1881–1887.
Publication types
MeSH terms
Substances
Grants and funding
- R01ES016746/ES/NIEHS NIH HHS/United States
- U19 AI070453/AI/NIAID NIH HHS/United States
- U19 ES019528/ES/NIEHS NIH HHS/United States
- RC2 ES018766/ES/NIEHS NIH HHS/United States
- R01 ES016746/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical