The PARL family of mitochondrial rhomboid proteases - PubMed (original) (raw)
Review
The PARL family of mitochondrial rhomboid proteases
R Blake Hill et al. Semin Cell Dev Biol. 2010 Aug.
Abstract
Rhomboids are an ancient and conserved family of intramembrane-cleaving proteases, a small group of proteolytic enzymes capable of hydrolyzing a peptide bond within a transmembrane helix that anchors a substrate protein to the membrane. Mitochondrial rhomboids evolved in eukaryotes to coordinate a critical aspect of cell biology, the regulation of mitochondrial membranes dynamics. This function appears to have required the emergence of a structural feature that is unique among all other rhomboids: an additional transmembrane helix (TMH) positioned at the N-terminus of six TMHs that form the core proteolytic domain of all prokaryotic and eukaryotic rhomboids. This "1+6" structure, which is shared only among mitochondrial rhomboids, defines a subfamily of rhomboids with the prototypical family member being mammalian Parl. Here, we present the findings that in 11 years have elevated mitochondrial rhomboids as the gatekeepers of mitochondrial dynamics and apoptosis; further, we discuss the aspects of their biology that are bound to introduce new paradigm shifts in our understanding of how the organelle uses this unique type of protease to govern stress, signaling to the nucleus, and other key mitochondrial activities in health and disease.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
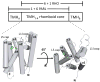
Figure 1
Domain architecture of rhomboid proteases. Rhomboids are composed of a 6 TMH core domain found in prokaryotes with two subfamilies that differ in the placement of an additional TMH at either the N-terminus (“1+6” mitochondrial PARL subfamily) or the C-terminus (“6+1” RHO subfamily). High resolution structures of the 6 TMH core rhomboid domain from bacterial rhomboids have been determined [12-15,35] and show the architecture of the active site with the absolutely conserved Ser and His arranged in a manner akin to classical serine proteases. Based on this and other evidence, Ser is thought to act as a nucleophile and initiate catalysis on the polypeptide substrate. Histidine plays essential acid-base roles in catalysis. The figure was made using the software PyMOL [110] and 3B45.pdb [35].
Similar articles
- Intramembrane proteolysis of Mgm1 by the mitochondrial rhomboid protease is highly promiscuous regarding the sequence of the cleaved hydrophobic segment.
Schäfer A, Zick M, Kief J, Steger M, Heide H, Duvezin-Caubet S, Neupert W, Reichert AS. Schäfer A, et al. J Mol Biol. 2010 Aug 13;401(2):182-93. doi: 10.1016/j.jmb.2010.06.014. Epub 2010 Jun 15. J Mol Biol. 2010. PMID: 20558178 - Rhomboid proteases in mitochondria and plastids: keeping organelles in shape.
Jeyaraju DV, Sood A, Laforce-Lavoie A, Pellegrini L. Jeyaraju DV, et al. Biochim Biophys Acta. 2013 Feb;1833(2):371-80. doi: 10.1016/j.bbamcr.2012.05.019. Epub 2012 May 24. Biochim Biophys Acta. 2013. PMID: 22634239 Review. - PARL Protease: A Glimpse at Intramembrane Proteolysis in the Inner Mitochondrial Membrane.
Lysyk L, Brassard R, Touret N, Lemieux MJ. Lysyk L, et al. J Mol Biol. 2020 Aug 21;432(18):5052-5062. doi: 10.1016/j.jmb.2020.04.006. Epub 2020 Apr 19. J Mol Biol. 2020. PMID: 32320686 Review. - Structural and mechanistic basis of Parl activity and regulation.
Jeyaraju DV, McBride HM, Hill RB, Pellegrini L. Jeyaraju DV, et al. Cell Death Differ. 2011 Sep;18(9):1531-9. doi: 10.1038/cdd.2011.22. Epub 2011 Mar 18. Cell Death Differ. 2011. PMID: 21415861 Free PMC article. - Self-regulated cleavage of the mitochondrial intramembrane-cleaving protease PARL yields Pbeta, a nuclear-targeted peptide.
Sík A, Passer BJ, Koonin EV, Pellegrini L. Sík A, et al. J Biol Chem. 2004 Apr 9;279(15):15323-9. doi: 10.1074/jbc.M313756200. Epub 2004 Jan 19. J Biol Chem. 2004. PMID: 14732705
Cited by
- Conformational change in rhomboid protease GlpG induced by inhibitor binding to its S' subsites.
Xue Y, Chowdhury S, Liu X, Akiyama Y, Ellman J, Ha Y. Xue Y, et al. Biochemistry. 2012 May 8;51(18):3723-31. doi: 10.1021/bi300368b. Epub 2012 Apr 24. Biochemistry. 2012. PMID: 22515733 Free PMC article. - Rhomboids of Mycobacteria: characterization using an aarA mutant of Providencia stuartii and gene deletion in Mycobacterium smegmatis.
Kateete DP, Katabazi FA, Okeng A, Okee M, Musinguzi C, Asiimwe BB, Kyobe S, Asiimwe J, Boom WH, Joloba ML. Kateete DP, et al. PLoS One. 2012;7(9):e45741. doi: 10.1371/journal.pone.0045741. Epub 2012 Sep 21. PLoS One. 2012. PMID: 23029216 Free PMC article. - Common variants of the PINK1 and PARL genes do not confer genetic susceptibility to schizophrenia in Han Chinese.
Li X, Zhang W, Zhang C, Yi Z, Zhang DF, Gong W, Tang J, Wang D, Lu W, Chen X, Fang Y, Yao YG. Li X, et al. Mol Genet Genomics. 2015 Apr;290(2):585-92. doi: 10.1007/s00438-014-0942-1. Epub 2014 Oct 30. Mol Genet Genomics. 2015. PMID: 25354644 - HRD Complex Self-Remodeling Enables a Novel Route of Membrane Protein Retrotranslocation.
Neal S, Syau D, Nejatfard A, Nadeau S, Hampton RY. Neal S, et al. iScience. 2020 Aug 21;23(9):101493. doi: 10.1016/j.isci.2020.101493. Online ahead of print. iScience. 2020. PMID: 32891886 Free PMC article. - Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL.
Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Jin SM, et al. J Cell Biol. 2010 Nov 29;191(5):933-42. doi: 10.1083/jcb.201008084. J Cell Biol. 2010. PMID: 21115803 Free PMC article.
References
- Tolia A, De Strooper B. Structure and function of gamma-secretase. Semin Cell Dev Biol. 2009;20:211–218. - PubMed
- Bork P, Koonin EV. Predicting functions from protein sequences--where are the bottlenecks? Nat Genet. 1998;18:313–318. - PubMed
- Koonin EV, Galperin MY. Sequence-evolution-function: Computational approaches in comparative genomics. Kluwer Academic Publishers Group; Dordrecht, The Netherlands: 2003. - PubMed
- Pellegrini L, Passer BJ, Canelles M, Lefterov I, Ganjei JK, Fowlkes BJ, Koonin EV, D'Adamio L. PAMP and PARL, two novel putative metalloproteases interacting with the COOH-terminus of Presenilin-1 and -2. J Alzheimers Dis. 2001;3:181–190. - PubMed
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, Derks C, Dejaegere T, Pellegrini L, D'Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM067180-05S1/GM/NIGMS NIH HHS/United States
- MOP-82718/CAPMC/ CIHR/Canada
- R01GM067180/GM/NIGMS NIH HHS/United States
- R01 GM067180-05/GM/NIGMS NIH HHS/United States
- R01 GM067180/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases