A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study - PubMed (original) (raw)
Clinical Trial
doi: 10.1186/1741-7015-8-3.
Helena Linardou, Gerasimos Aravantinos, Christos Papadimitriou, Aristotelis Bamias, George Fountzilas, Haralabos P Kalofonos, Paris Kosmidis, Eleni Timotheadou, Thomas Makatsoris, Epaminondas Samantas, Evangelos Briasoulis, Christos Christodoulou, Pavlos Papakostas, Dimitrios Pectasides, Athanasios M Dimopoulos
Affiliations
- PMID: 20055981
- PMCID: PMC2823653
- DOI: 10.1186/1741-7015-8-3
Clinical Trial
A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study
Dimitrios Bafaloukos et al. BMC Med. 2010.
Abstract
Background: Platinum-based combinations are the standard second-line treatment for platinum-sensitive ovarian cancer (OC). This randomized phase II study was undertaken in order to compare the combination of carboplatin and pegylated liposomal doxorubicin (LD) with carboplatin and paclitaxel (CP) in this setting.
Methods: Patients with histologically confirmed recurrent OC, at the time of or more than 6 months after platinum-based chemotherapy, were randomized to six cycles of CP (carboplatin AUC5 + paclitaxel 175 mg/m2, d1q21) or CLD (carboplatin AUC5 + pegylated LD 45 mg/m2, d1q28).
Results: A total of 189 eligible patients (CP 96, CLD 93), with a median age of 63 years, median Performance Status (PS) 0 and a median platinum free interval (PFI) of 16.5 months, entered the study. Discontinuation due to toxicity was higher in the CP patients (13.5% versus 3%, P = 0.016). The overall response rate was similar: CP 58% versus CLD 51%, P = 0.309 (Complete Response; CR 34% versus 23%) and there was no statistical difference in time-to-progression (TTP) or overall survival (OS; TTP 10.8 months CP versus 11.8 CLD, P = 0.904; OS 29.4 months CP versus 24.7 CLD, P = 0.454). No toxic deaths were recorded. Neutropenia was the most commonly seen severe toxicity (CP 30% versus CLD 35%). More frequent in CLD were severe thrombocytopenia (11% versus 2%, P = 0.016), skin toxicity and Palmar-plantar erythrodysesthesia (PPE) grade 1-2 (38% versus 9%, P< 0.001), while grade 3 neurotoxicity and alopecia were higher in CP (7% versus 0%, P = 0.029, 20% versus 5%, P = 0.003). PS and PFI were independent prognostic factors for TTP and OS.
Conclusions: The combination of pegylated LD with carboplatin is effective, showing less neurotoxicity and alopecia than paclitaxel-carboplatin. It thus warrants a further phase III evaluation as an alternative treatment option for platinum-sensitive OC patients.
Trial registration: Australian New Zealand Clinical Trials Registry: ACTRN12609000436279.
Figures
Figure 1
Treatment schema.
Figure 2
Progress through the various stages of the trial.
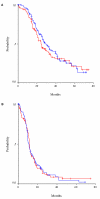
Figure 3
Kaplan-Meier curves for (A) overall survival, (B) time to progression. The blue line corresponds to group A, while the red line to group B.
Similar articles
- Pegylated liposomal doxorubicin for first-line treatment of epithelial ovarian cancer.
Lawrie TA, Rabbie R, Thoma C, Morrison J. Lawrie TA, et al. Cochrane Database Syst Rev. 2013 Oct 21;2013(10):CD010482. doi: 10.1002/14651858.CD010482.pub2. Cochrane Database Syst Rev. 2013. PMID: 24142521 Free PMC article. Review. - Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial.
Mahner S, Meier W, du Bois A, Brown C, Lorusso D, Dell'Anna T, Cretin J, Havsteen H, Bessette P, Zeimet AG, Vergote I, Vasey P, Pujade-Lauraine E, Gladieff L, Ferrero A. Mahner S, et al. Eur J Cancer. 2015 Feb;51(3):352-8. doi: 10.1016/j.ejca.2014.11.017. Epub 2014 Dec 17. Eur J Cancer. 2015. PMID: 25534295 Clinical Trial. - Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum-sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial.
Gladieff L, Ferrero A, De Rauglaudre G, Brown C, Vasey P, Reinthaller A, Pujade-Lauraine E, Reed N, Lorusso D, Siena S, Helland H, Elit L, Mahner S. Gladieff L, et al. Ann Oncol. 2012 May;23(5):1185-1189. doi: 10.1093/annonc/mdr441. Epub 2011 Oct 5. Ann Oncol. 2012. PMID: 21976386 Clinical Trial. - Safety of a 3-weekly schedule of carboplatin plus pegylated liposomal doxorubicin as first line chemotherapy in patients with ovarian cancer: preliminary results of the MITO-2 randomized trial.
Pignata S, Scambia G, Savarese A, Breda E, Scollo P, De Vivo R, Rossi E, Gebbia V, Natale D, Del Gaizo F, Naglieri E, Ferro A, Musso P, D'Arco AM, Sorio R, Pisano C, Di Maio M, Signoriello G, Annunziata A, Perrone F; MITO Investigators. Pignata S, et al. BMC Cancer. 2006 Aug 1;6:202. doi: 10.1186/1471-2407-6-202. BMC Cancer. 2006. PMID: 16882344 Free PMC article. Clinical Trial. - Tolerability, efficacy, and safety of pegylated liposomal Doxorubicin in combination with Carboplatin versus gemcitabine-Carboplatin for the treatment of platinum-sensitive recurrent ovarian cancer: a systematic review.
Holloway RW, Grendys EC, Lefebvre P, Vekeman F, McMeekin S. Holloway RW, et al. Oncologist. 2010;15(10):1073-82. doi: 10.1634/theoncologist.2009-0331. Epub 2010 Oct 7. Oncologist. 2010. PMID: 20930103 Free PMC article.
Cited by
- A comprehensive comparison of medication strategies for platinum-sensitive recurrent ovarian cancer: A Bayesian network meta-analysis.
Liu Y, Huang Y, Li J, Wan S, Jiang N, Yang J, Chiampanichayakul S, Tima S, Anuchapreeda S, Wu J. Liu Y, et al. Front Pharmacol. 2022 Nov 10;13:1010626. doi: 10.3389/fphar.2022.1010626. eCollection 2022. Front Pharmacol. 2022. PMID: 36438821 Free PMC article. - Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients.
Wagner U, Marth C, Largillier R, Kaern J, Brown C, Heywood M, Bonaventura T, Vergote I, Piccirillo MC, Fossati R, Gebski V, Lauraine EP. Wagner U, et al. Br J Cancer. 2012 Aug 7;107(4):588-91. doi: 10.1038/bjc.2012.307. Epub 2012 Jul 26. Br J Cancer. 2012. PMID: 22836511 Free PMC article. Clinical Trial. - Efficacy and safety of traditional chemotherapies for patients with ovarian neoplasm: a network meta-analysis.
Yang L, Guo G, Sun L, Li C, Zhang H. Yang L, et al. Oncotarget. 2017 Mar 30;8(35):59867-59877. doi: 10.18632/oncotarget.16729. eCollection 2017 Aug 29. Oncotarget. 2017. PMID: 28938689 Free PMC article. Review. - Pegylated liposomal doxorubicin for first-line treatment of epithelial ovarian cancer.
Lawrie TA, Rabbie R, Thoma C, Morrison J. Lawrie TA, et al. Cochrane Database Syst Rev. 2013 Oct 21;2013(10):CD010482. doi: 10.1002/14651858.CD010482.pub2. Cochrane Database Syst Rev. 2013. PMID: 24142521 Free PMC article. Review. - Pegylated liposomal doxorubicin in the management of ovarian cancer: a systematic review and metaanalysis of randomized trials.
Staropoli N, Ciliberto D, Botta C, Fiorillo L, Grimaldi A, Lama S, Caraglia M, Salvino A, Tassone P, Tagliaferri P. Staropoli N, et al. Cancer Biol Ther. 2014 Jun 1;15(6):707-20. doi: 10.4161/cbt.28557. Epub 2014 Mar 21. Cancer Biol Ther. 2014. PMID: 24658024 Free PMC article. Review.
References
- American Cancer Society. Cancer Facts and Figures 2007. Georgia: American Cancer Society; 2007.
- du Bois A, Lück HJ, Meier W, Adams HP, Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1330. - PubMed
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous