Genetic control of active neural circuits - PubMed (original) (raw)
Genetic control of active neural circuits
Leon Reijmers et al. Front Mol Neurosci. 2009.
Abstract
The use of molecular tools to study the neurobiology of complex behaviors has been hampered by an inability to target the desired changes to relevant groups of neurons. Specific memories and specific sensory representations are sparsely encoded by a small fraction of neurons embedded in a sea of morphologically and functionally similar cells. In this review we discuss genetics techniques that are being developed to address this difficulty. In several studies the use of promoter elements that are responsive to neural activity have been used to drive long-lasting genetic alterations into neural ensembles that are activated by natural environmental stimuli. This approach has been used to examine neural activity patterns during learning and retrieval of a memory, to examine the regulation of receptor trafficking following learning and to functionally manipulate a specific memory trace. We suggest that these techniques will provide a general approach to experimentally investigate the link between patterns of environmentally activated neural firing and cognitive processes such as perception and memory.
Keywords: amygdala; creb; fear conditioning; fos; genetics; memory; neural circuits; tetracycline.
Figures
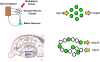
Figure 1
Clustered and distributed neural ensembles. Two examples of neural circuits are shown. The top panel shows a simplified version of the gill and siphon withdrawal circuit in Aplysia. The sensory neuron cell bodies are located adjacent to each other in a cluster and possess similar biochemistry and response properties. The bottom panel represents the hippocampal circuit of the mammalian brain. The green circles represent neurons that are activated by a specific pattern of sensory stimulation and that when activated contribute to a specific behavioral response. The hippocampal circuit, like many other circuits in the brain, responds to sensory stimulation with activation patterns that can not be predicted from their wiring diagram. Each of these neural ensembles involves a sparse subset of neurons that have an unpredictable spatial distribution. The Aplysia neurons are primary sensory neurons and their response properties can be predicted by their physical location in the ganglion.
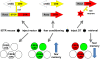
Figure 2
The TetTag mouse. The figure summarizes an experiment from Reijmers et al. (2007). Mice carrying two transgenes were used. The first transgene uses the cfos promoter to drive expression of the tetracycline transactivator (tTA). tTA activates the tetO promoter in the absence but not presence of doxycycline (Dox). The second transgene uses the tetO promoter to drive expression of a Dox insensitive tTA (tTA*), which, once expressed, sets up a positive feedback loop that continuously drives expression of a β-galactosidase reporter coupled to the tau protein (taulacZ). Neurons activated during fear conditioning (while off Dox) were tagged with long-lasting expression of taulacZ (LAC; red circle). Mice were put back on food with doxycycline and a retrieval test was done 3 days later, followed by analysis of the brains 1 h after retrieval for expression of lacZ and zif268. Neurons activated during learning expressed lacZ and those active during retrieval expressed zif268 (ZIF; green circle). The number of neurons in the amygdala that expressed both LAC and ZIF, indicating that they were activate during both learning and retrieval, was positively correlated with the strength of the fear memory that the animal displayed.
Figure 3
Disrupting a specific memory in the mouse. The figure summarizes the experiments of Han et al. (2009). Transgenic mice were used that express an inducible diphtheria toxin receptor (iDTR). A viral vector expressing both CREB and CRE recombinase was injected into the amygdala of iDTR mice leading to the expression of both CRE and CREB in a random subset of neurons (circles with CREB/CRE). The CRE recombinase removed a transcriptional STOP sequence and allowed for expression of DTR in these CREB expressing neurons. The mice were then subjected to fear conditioning. An earlier study from the same authors (Han et al., 2007) demonstrated that the CREB expressing neurons participate in the storage of the fear memory (green circles symbolize neurons that participate in the storage of the memory). After fear conditioning, mice were injected with diphtheria toxin (DT), which killed the CREB expressing neurons which participated in the encoding of that memory (red circles). This caused a significant reduction in the strength of the fear memory measured during retrieval.
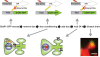
Figure 4
Learning regulated targeting of glutamate receptors. The figure summarizes an experiment from Matsuo et al. (2008). Mice carrying two transgenes were used. The first transgene is identical to the one described in Figure 2 and uses the cfos promoter to drive expression of a tetracycline transactivator (tTA). The second transgene was a tetO-promoter GFP tagged glutamate receptor subunit (GluR1-GFP). Animals were fear conditioned in the absence of Dox to produce both a fear memory and a pulse of GFP-GluR1 expression in active neural ensembles in the hippocampus. The distribution of GFP-GluR1 in dendritic spines was analyzed 24 h following the conditioning using DiI to label all spines on a given neuron. Fear conditioning led to an increase in trafficking of the receptor specifically to mushroom type spines. This experiment demonstrates how genetic tools can be used to image a specific molecular event selectively within an activated neural circuit.
Similar articles
- Amygdala kindling disrupts trace and delay fear conditioning with parallel changes in Fos protein expression throughout the limbic brain.
Botterill JJ, Fournier NM, Guskjolen AJ, Lussier AL, Marks WN, Kalynchuk LE. Botterill JJ, et al. Neuroscience. 2014 Apr 18;265:158-71. doi: 10.1016/j.neuroscience.2014.01.040. Epub 2014 Jan 31. Neuroscience. 2014. PMID: 24486965 - β-Amyloid pathology alters neural network activation during retrieval of contextual fear memories in a mouse model of Alzheimer's disease.
Lelos MJ, Good MA. Lelos MJ, et al. Eur J Neurosci. 2014 May;39(10):1690-703. doi: 10.1111/ejn.12527. Epub 2014 Mar 16. Eur J Neurosci. 2014. PMID: 24628842 - Bidirectional Modulation of Intrinsic Excitability in Rat Prelimbic Cortex Neuronal Ensembles and Non-Ensembles after Operant Learning.
Whitaker LR, Warren BL, Venniro M, Harte TC, McPherson KB, Beidel J, Bossert JM, Shaham Y, Bonci A, Hope BT. Whitaker LR, et al. J Neurosci. 2017 Sep 6;37(36):8845-8856. doi: 10.1523/JNEUROSCI.3761-16.2017. Epub 2017 Aug 4. J Neurosci. 2017. PMID: 28779019 Free PMC article. - Exploring Memory Representations with Activity-Based Genetics.
Mayford M, Reijmers L. Mayford M, et al. Cold Spring Harb Perspect Biol. 2015 Dec 18;8(3):a021832. doi: 10.1101/cshperspect.a021832. Cold Spring Harb Perspect Biol. 2015. PMID: 26684182 Free PMC article. Review. - Illuminating the Activated Brain: Emerging Activity-Dependent Tools to Capture and Control Functional Neural Circuits.
He Q, Wang J, Hu H. He Q, et al. Neurosci Bull. 2019 Jun;35(3):369-377. doi: 10.1007/s12264-018-0291-x. Epub 2018 Sep 26. Neurosci Bull. 2019. PMID: 30255458 Free PMC article. Review.
Cited by
- Distinct Populations of Neurons Activated by Heroin and Cocaine in the Striatum as Assessed by catFISH.
Vassilev P, Avvisati R, Koya E, Badiani A. Vassilev P, et al. eNeuro. 2020 Feb 6;7(1):ENEURO.0394-19.2019. doi: 10.1523/ENEURO.0394-19.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31937522 Free PMC article. - Tools for resolving functional activity and connectivity within intact neural circuits.
Jennings JH, Stuber GD. Jennings JH, et al. Curr Biol. 2014 Jan 6;24(1):R41-R50. doi: 10.1016/j.cub.2013.11.042. Curr Biol. 2014. PMID: 24405680 Free PMC article. Review. - Applying an Inducible Expression System to Study Interference of Bacterial Virulence Factors with Intracellular Signaling.
Berens C, Bisle S, Klingenbeck L, Lührmann A. Berens C, et al. J Vis Exp. 2015 Jun 25;(100):e52903. doi: 10.3791/52903. J Vis Exp. 2015. PMID: 26168006 Free PMC article. - The emergence of molecular systems neuroscience.
Shen Y, Luchetti A, Fernandes G, Do Heo W, Silva AJ. Shen Y, et al. Mol Brain. 2022 Jan 4;15(1):7. doi: 10.1186/s13041-021-00885-5. Mol Brain. 2022. PMID: 34983613 Free PMC article. Review. - _Arc-_Expressing Accessory Olfactory Bulb Interneurons Support Chemosensory Social Behavioral Plasticity.
Zuk KE, Cansler HL, Wang J, Meeks JP. Zuk KE, et al. J Neurosci. 2023 Feb 15;43(7):1178-1190. doi: 10.1523/JNEUROSCI.0847-22.2022. Epub 2023 Jan 9. J Neurosci. 2023. PMID: 36623874 Free PMC article.
References
- Alexander G. M., Rogan S. C., Abbas A. I., Armbruster B. N., Pei Y., Allen J. A., Nonneman R. J., Hartmann J., Moy S. S., Nicolelis M. A., McNamara J. O., Roth B. L. (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–3910.1016/j.neuron.2009.06.014 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources