Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits - PubMed (original) (raw)
doi: 10.1016/j.devcel.2009.11.005.
Andrea Raimondi, Summer Paradise, Hongying Shen, Kumi Mesaki, Agnes Ferguson, Olivier Destaing, Genevieve Ko, Junko Takasaki, Ottavio Cremona, Eileen O' Toole, Pietro De Camilli
Affiliations
- PMID: 20059951
- PMCID: PMC2861561
- DOI: 10.1016/j.devcel.2009.11.005
Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits
Shawn M Ferguson et al. Dev Cell. 2009 Dec.
Erratum in
- Dev Cell. 2010 Feb 16;18(2):332. Ferguson, Shawn [corrected to Ferguson, Shawn M]
Abstract
The GTPase dynamin, a key player in endocytic membrane fission, interacts with numerous proteins that regulate actin dynamics and generate/sense membrane curvature. To determine the functional relationship between these proteins and dynamin, we have analyzed endocytic intermediates that accumulate in cells that lack dynamin (derived from dynamin 1 and 2 double conditional knockout mice). In these cells, actin-nucleating proteins, actin, and BAR domain proteins accumulate at the base of arrested endocytic clathrin-coated pits, where they support the growth of dynamic long tubular necks. These results, which we show reflect the sequence of events in wild-type cells, demonstrate a concerted action of these proteins prior to, and independent of, dynamin and emphasize similarities between clathrin-mediated endocytosis in yeast and higher eukaryotes. Our data also demonstrate that the relationship between dynamin and actin is intimately connected to dynamin's endocytic role and that dynamin terminates a powerful actin- and BAR protein-dependent tubulating activity.
2009 Elsevier Inc. All rights reserved.
Figures
Figure 1
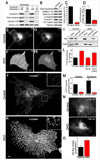
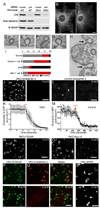
Cellular proliferation and clathrin mediated endocytosis defects in the absence of dynamin. (A) Immunoblotting analysis with isoform specific antibodies shows that depletion of total dynamin levels from fibroblast cultures requires the KO of both dynamin 1 and dynamin 2. Cells were harvested at 7 days post-transduction with either a control or a Cre recombinase retrovirus. (B) Tamoxifen treatment of Dnm1LoxP/LoxP;Dnm2LoxP/LoxP;CRE-ER+/0 cells depleted total levels of all dynamin proteins, levels of clathrin-coated pit proteins were unaffected, caveolin 1 levels were reduced and levels of acetylated tubulin (Ac-tubulin) were increased. Results are representative of >4 independent experiments. (C) BrDU incorporation in control versus DKO cells (p=0.014, t-test, n=3 experiments). (D) Dynamin DKO cells exhibit a proliferation defect as revealed by cell counting (p=0.0013, t-test, n=3 experiments). (E) and (F) Uptake of Alexa594 labeled transferrin (Tf) in a representative control cell (E) and DKO cell (F) showing strong intracellular/endosomal versus plasma membrane localizations respectively. (G) and (H) Transferrin receptor(TfR)-pHluorin is predominantly localized in endosomes in control cells (G) and at cell surface in DKO cells (H). (I) Compared to control cells, DKO cells have highly elevated levels of endogenously expressed transferrin receptor in the plasma membrane/biotinylated fraction. (J) and (K) Immunofluorescence (epifluorescence microscopy) staining for the endocytic clatrhin coated pit marker α-adaptin reveals a higher density of pits in the control (J) than in the DKO cell (K). The insets show higher magnifications of the boxed regions. Note that the highly abundant clathrin-coated pits in the DKO cell (K) tend to cluster together. (L) Electroporation with dynamin 2-GFP (Dyn2) but not dynamin 2-ΔPRD-GFP rescued the DKO clathrin-coated pit abundance phenotype. Quantification based on α-adaptin immunostaining (mean+/−SEM, n=12–14 cells/condition). (M) Rates of clathrin-coated pit appearance and disappearance measured from spinning disk confocal imaging of µ2-GFP in Control and DKO cells (n=6 cells/genotype). (N) Representative images of control and DKO cells following uptake of FM1-43 (epifluorescence microscopy). (O) Quantification of FM1-43 uptake (n=10 control and 11 DKO cells respectively). Scale bars represent 10µm. Data are presented as mean+/−SEM. See also Figs. S1 and S2 and Movie S1 for supporting data.
Figure 2
Massive tubulation of plasma membrane clathrin-coated pits. Representative transmission electron microscopy images of shallow (A), omega-shaped (B) and tubulated clathrin-coated pits (C–E) and tubule fragments (F and G) in DKO cells (~60nm thick sections). Arrows in (E and G) point to cross-sectioned tubules. Arrowheads in F point to the dense cytoskeletal matrix that surrounds a cross-sectioned tubule. The scale bar in panel A=100 nm and applies to panels A–G. (H) Morphometric analysis of electron microscopy images demonstrated the accumulation of tubulated clathrin-coated pits and tubule fragments (t test, p=0.002 and p=0.046 respectively) within 500 nm of the plasma membrane of DKO cells. Inset shows sketches of the structures included in each category. Data was obtained from 3 independent experiments. > 10 cell profiles per genotype were randomly selected for analysis in each experiment. (I) The great abundance of narrow tubules just below the DKO cell surface is evident in this Z-axis maximal projection of 90 slices from a dual axis tomographic reconstruction representing a 180 nm thick sub-volume from the original 250 nm thick section. (J) Electron tomography 3-dimensional reconstruction from single axis tomograms obtained from 3 serial DKO sections (~750 nm total thickness) illustrating the profusion of long, narrow plasma membrane tubules (green) most (13 of 16) of which are capped by clathrin-coated pits (red). Scale bars in (I) and (J) =250 nm. Data are presented as mean+/−SEM.
Figure 3
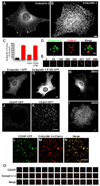
Dynamin binding BAR domain proteins accumulate at the neck of clathrin-coated pits in DKO cells. (A and B) Endophilin 2 immunoreactivity in control and DKO cells respectively (epifluorescence microscopy). (C) Electroporation with dynamin 2-GFP (Dyn2) but not dynamin 2-ΔPRD-GFP rescued the phenotype of endophilin 2 clustering. Quantification based on immunostaining of endogenous endophilin 2 protein (n=10–21 cells/condition). (D) Spinning disk confocal imaging of endophilin 2-GFP and mRFP-clathrin light chain (LCa) showing the presence of endophilin 2-positive tubules at the base of a cluster of clathrin-coated pits in a DKO cell. (E) Time course showing sequential recruitment of mRFP-LCa (red) and endophilin 2-GFP (green) in a DKO cell. Numbers refer to the relative time in seconds at which each image was taken. (F) and (G) Endophilin 1-GFP (F) but not the endophilin 1-F10E-mutant (G) exhibits a punctate pattern of localization in DKO cells. (H) and (I) Immunofluorescence staining of endogenous SNX9 in control and DKO cells respectively. (J) and (K) CD2AP-GFP localization pattern in control and DKO cells respectively. (L–N) Co-localization of CD2AP-GFP with endophilin 2-mCherry at the plasma membrane of a DKO cell. (O) Sequence of CD2AP-GFP and endophilin 2-mCherry recruitment to a clathrin-coated pit in a control cell. Frames were taken at 4 second intervals. Panels A, B, F–I are epifluorescence microscopy images, the remainder are spinning disk confocal images. Scale bars for A, B, F–K= 10µm, D and E = 1µm and L–N = 5µm. Data are presented as mean+/−SEM. See also Fig. S3 for and Movies S2 and S5 for supplementary data.
Figure 4
Enrichment of F-actin at clathrin-coated pits in DKO cells (A and B) Phalloidin staining in control and DKO cells respectively. (C and D) Labeling of the same pair of cells from A and B with anti-Arp2/3 (p34 subunit) antibodies. (E) Merged image of phalloidin (left and red in merged image) and Arp2/3 (middle panel, green in merge) staining from boxed region highlighted in B and D. (F) Quantification of the abundance of Arp2/3 puncta and rescue of the DKO phenotype by dynamin 2 but not dynamin 2ΔPRD (n=9–13 cells/condition). (G) Clathrin-coated pits (µ2-GFP reporter) are sites of F-actin (Utr-CH-mCherry reporter) enrichment in DKO cells. (H) Endophilin 2-GFP and Utr-CH-mCherry co-localize in DKO cells. (I) Control cell double labeled for F-actin (phalloidin staining) and endogenous endophilin 2 (red=F-actin, green=endophilin 2). (J) Co-localization of F-actin (phalloidin staining) and endogenous endophilin 2 foci in a representative DKO cell (red=F-actin, green=endophilin 2). (K) F-actin (Utr-CH-mCherry) recruitment coincides with clathrin-coated pit (µ2-GFP) disappearance in a control cell. Images taken at 4 second intervals. Scale bars = 10 µm in B, D and H and 5 µm in J. Images in A–E, G and H were acquired by epifluorescence microscopy and by spinning disk confocal in I–K. Data are presented as mean+/−SEM. Supporting data is presented in Figure S4.
Figure 5
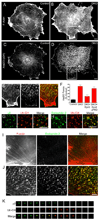
Clathrin is required for the endophilin and actin phenotypes of DKO cells. (A) Transfection of clathrin heavy chain (CHC) siRNA effectively suppresses clathrin heavy chain protein levels in both wildtype and DKO cells (5 days post-transfection). (B) Suppression of clathrin heavy chain expression depletes clathrin-coated pits from DKO cells as assessed by clathrin light chain immunofluorescence. (C) Endophilin 2, (D) cortactin, (E) Arp2/3 (p34 subunit) and (F) F-actin no longer accumulate in clusters at the plasma membrane of DKO cells lacking CHC. The remaining Arp2/3 puncta are comparable in abundance to those observed in control cells (see Fig. 3E). The 10µm scale bar in panel B applies to B–D. The 10µm scale bar in panel F applies to both E and F. All images were acquired by epifluorescence microscopy.
Figure 6
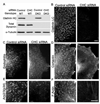
Tubulation of clathrin-coated pits is acutely dependent on actin polymerization. (A) siRNA-mediated depletion of Arp2/3 complex p34 subunit in control and DKO cells (3 days after transfection). (B) and (C) Representative endophilin 2 immunostaining from control siRNA transfected and Arp2/3 (p34) siRNA transfected DKO cells respectively. (D) Representative example of a clathrin-coated pit from a control cell under basal conditions. (E) Example demonstrating a clathrin-coated pit with a widened neck from a control cell that was fixed after 90 seconds of treatment with 5 µM latrunculin B (Lat. B). (F) A tubulated clathrin-coated pit that is typical of DKO cells under basal conditions. (G and H) Following Lat. B treatment, clathrin-coated pits in DKO cells no longer have long, narrow, tubular necks but are instead connected to the plasma membrane by short, wide necks. Arrows in (H) point to 2 such pits. (I) Quantification of endocytic clathrin-coated pit morphology in control and DKO cells both before and after latrunculin B treatment. The stacked bars show the percentage of pit structures represented by each morphology under the indicated conditions. Data represents 106 control and 266 DKO clathrin-coated pits randomly identified from 3 independent experiments. (J) Endophilin 2-GFP puncta in DKO cells are dispersed upon Lat. B treatment. (K) Quantification of time lapse imaging of changes in endophilin 2-GFP spot abundance in DKO cells upon Lat. B administration (τ= 56.0 sec, n=8 cells). (L and M) Endophilin 2-GFP recruitment is also Lat. B sensitive in control cells (τ= 30.1 sec, n=11 cells). (N and O) The clustering of CD2AP-GFP and Myo1E-GFP in DKO cells are sensitive to Lat. B. (P–R) GFP-N-WASP (P) becomes brighter and more punctate in DKO cells in response to actin depolymerization with Lat. B while the co-expressed endophilin 2-mCherry (Q) that initially co-localized with N-WASP dispersed. (S) GFP-SNX9 in DKO cells lost its tubular localization and became punctate in response to Lat. B. All representative images of Lat. B treatment (5 µM) correspond a time point 90 seconds after addition of the drug. Scale bars = 10µm in B and C, 100nm in D–H and 2.5 µm in J–S. Images in B and C are maximal projections of deconvolved spinning disk confocal Z-stacks. Images in J–L and N–S are single optical sections from spinning disk confocal microscopy. Data are presented as mean+/−SEM. Data in this figure are further supported by Movies S3–S9.
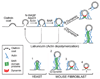
Figure 7
Diagrams illustrating the sequence of events at mammalian endocytic clathrin coated pits and the evolutionarily conserved relationship between yeast actin patches and clathrin coated pits of higher eukaryotes. Top: Proposed relationship between recruitment of various factors and maturation of endocytic clathrin-coated pits. Dynamin mediates, or contributes to, membrane fission by acting on a tubular template that is generated by growth of the coat and that is further constricted and elongated by a concerted action of actin and BAR proteins. The dynamically extending and contracting tubules observed in the absence of dynamin represent the overgrowth of such templates. Disruption of F-actin by latrunculin leads to collapse of the tubules to a short and wide clathrin-coated pit neck. Bottom: Comparison of clathrin-coated endocytic intermediates and of the molecular machinery supporting their formation in yeast (adapted from Kaksonen et al, 2006) and in mouse fibroblasts. The presence of dynamin in mouse cells (3), but not in yeast (1), minimizes tubule elongation. Lack of dynamin (2) reveals the similarity of events upstream of fission in yeast and fibroblasts. The scenario shown in (4) reflects the reported occurrence of actin-independent clathrin-mediated endocytic events. The membrane curvature sensing properties of BAR proteins and/or the direct or indirect interactions of several BAR proteins with components of the clathrin coat may be sufficient to trigger dynamin recruitment to endocytic pits under such conditions.
Similar articles
- Constitutive activated Cdc42-associated kinase (Ack) phosphorylation at arrested endocytic clathrin-coated pits of cells that lack dynamin.
Shen H, Ferguson SM, Dephoure N, Park R, Yang Y, Volpicelli-Daley L, Gygi S, Schlessinger J, De Camilli P. Shen H, et al. Mol Biol Cell. 2011 Feb 15;22(4):493-502. doi: 10.1091/mbc.E10-07-0637. Epub 2010 Dec 17. Mol Biol Cell. 2011. PMID: 21169560 Free PMC article. - SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis.
Soulet F, Yarar D, Leonard M, Schmid SL. Soulet F, et al. Mol Biol Cell. 2005 Apr;16(4):2058-67. doi: 10.1091/mbc.e04-11-1016. Epub 2005 Feb 9. Mol Biol Cell. 2005. PMID: 15703209 Free PMC article. - A feedback loop between dynamin and actin recruitment during clathrin-mediated endocytosis.
Taylor MJ, Lampe M, Merrifield CJ. Taylor MJ, et al. PLoS Biol. 2012;10(4):e1001302. doi: 10.1371/journal.pbio.1001302. Epub 2012 Apr 10. PLoS Biol. 2012. PMID: 22505844 Free PMC article. - Dynamin rings: not just for fission.
Sever S, Chang J, Gu C. Sever S, et al. Traffic. 2013 Dec;14(12):1194-9. doi: 10.1111/tra.12116. Epub 2013 Sep 19. Traffic. 2013. PMID: 23980695 Free PMC article. Review. - Regulating dynamin dynamics during endocytosis.
Sundborger AC, Hinshaw JE. Sundborger AC, et al. F1000Prime Rep. 2014 Oct 1;6:85. doi: 10.12703/P6-85. eCollection 2014. F1000Prime Rep. 2014. PMID: 25374663 Free PMC article. Review.
Cited by
- The molecular organization of differentially curved caveolae indicates bendable structural units at the plasma membrane.
Matthaeus C, Sochacki KA, Dickey AM, Puchkov D, Haucke V, Lehmann M, Taraska JW. Matthaeus C, et al. Nat Commun. 2022 Nov 24;13(1):7234. doi: 10.1038/s41467-022-34958-3. Nat Commun. 2022. PMID: 36433988 Free PMC article. - The first five seconds in the life of a clathrin-coated pit.
Cocucci E, Aguet F, Boulant S, Kirchhausen T. Cocucci E, et al. Cell. 2012 Aug 3;150(3):495-507. doi: 10.1016/j.cell.2012.05.047. Cell. 2012. PMID: 22863004 Free PMC article. - Tissue-specific dynamin-1 deletion at the calyx of Held decreases short-term depression through a mechanism distinct from vesicle resupply.
Mahapatra S, Fan F, Lou X. Mahapatra S, et al. Proc Natl Acad Sci U S A. 2016 May 31;113(22):E3150-8. doi: 10.1073/pnas.1520937113. Epub 2016 May 16. Proc Natl Acad Sci U S A. 2016. PMID: 27185948 Free PMC article. - Myosin 1E coordinates actin assembly and cargo trafficking during clathrin-mediated endocytosis.
Cheng J, Grassart A, Drubin DG. Cheng J, et al. Mol Biol Cell. 2012 Aug;23(15):2891-904. doi: 10.1091/mbc.E11-04-0383. Epub 2012 Jun 6. Mol Biol Cell. 2012. PMID: 22675027 Free PMC article. - Reducing dynamin 2 (DNM2) rescues _DNM2_-related dominant centronuclear myopathy.
Buono S, Ross JA, Tasfaout H, Levy Y, Kretz C, Tayefeh L, Matson J, Guo S, Kessler P, Monia BP, Bitoun M, Ochala J, Laporte J, Cowling BS. Buono S, et al. Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):11066-11071. doi: 10.1073/pnas.1808170115. Epub 2018 Oct 5. Proc Natl Acad Sci U S A. 2018. PMID: 30291191 Free PMC article.
References
- Cao H, Thompson HM, Krueger EW, McNiven MA. Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J Cell Sci. 2000;113(Pt 11):1993–2002. - PubMed
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
- Cook T, Mesa K, Urrutia R. Three dynamin-encoding genes are differentially expressed in developing rat brain. J Neurochem. 1996;67:927–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR-000592/RR/NCRR NIH HHS/United States
- P01 CA046128/CA/NCI NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01 NS036251-12/NS/NINDS NIH HHS/United States
- R01 NS036251-11/NS/NINDS NIH HHS/United States
- DK45735/DK/NIDDK NIH HHS/United States
- GGP05141/TI_/Telethon/Italy
- CA46128/CA/NCI NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R01 NS036251/NS/NINDS NIH HHS/United States
- DA018343/DA/NIDA NIH HHS/United States
- P41 RR000592/RR/NCRR NIH HHS/United States
- NS36251/NS/NINDS NIH HHS/United States
- R37 NS036251/NS/NINDS NIH HHS/United States
- R01 NS036251-10/NS/NINDS NIH HHS/United States
- P01 CA046128-20/CA/NCI NIH HHS/United States
- P30 DK045735-119008/DK/NIDDK NIH HHS/United States
- P30 DA018343/DA/NIDA NIH HHS/United States
- R01 NS036251-13/NS/NINDS NIH HHS/United States
- P30 DA018343-06/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases