Syntaxin 3B is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina - PubMed (original) (raw)
Syntaxin 3B is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina
L Curtis et al. Neuroscience. 2010.
Abstract
Ribbon synapses of the vertebrate retina are specialized synapses that release neurotransmitter by synaptic vesicle exocytosis in a manner that is proportional to the level of depolarization of the cell. This release property is different from conventional neurons, in which the release of neurotransmitter occurs as a short-lived burst triggered by an action potential. Synaptic vesicle exocytosis is a calcium regulated process that is dependent on a set of interacting synaptic proteins that form the so-called SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor) complex. Syntaxin 3B has been identified as a specialized SNARE molecule in ribbon synapses of the rodent retina. However, the best physiologically-characterized neuron that forms ribbon-style synapses is the rod-dominant or Mb1 bipolar cell of the goldfish retina. We report here the molecular characterization of syntaxin 3B from the goldfish retina. Using a combination of reverse transcription (RT) polymerase chain reaction (PCR) and immunostaining with a specific antibody, we show that syntaxin 3B is highly enriched in the plasma membrane of bipolar cell synaptic terminals of the goldfish retina. Using membrane capacitance measurements we demonstrate that a peptide derived from goldfish syntaxin 3B inhibits synaptic vesicle exocytosis. These experiments demonstrate that syntaxin 3B is an important factor for synaptic vesicle exocytosis in ribbon synapses of the vertebrate retina.
Keywords: SNAP-25; SNARE; Synaptobrevin; VAMP; isoform; splicing; vesicle fusion.
Copyright 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
Fig. 1
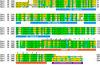
Sequence alignment of the different syntaxin isoforms. The protein sequences of the goldfish (CA) syntaxin 3B, zebrafish (DR) syntaxin 3B and the mouse (MM) syntaxin 3B and syntaxin 1A have been aligned for maximal homology using CLUSTALW. Sequences are identified on the left and residues numbered on the right. Residues that are conserved in all four proteins are labeled with green background. Residues conserved between three of the syntaxin isoforms are labeled with yellow background. Conservative exchanged residues are labeled with blue background. The positions of the conserved domains are marked below the sequence. The positions of the hydrophobic interacting layers are numbered in relation to the glutamine (Q) of the central 0 layer. The position of the peptide sequence used for the electrophysiological experiments is marked with a bar above the sequence.
Fig. 2
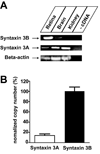
Expression of Syntaxin 3B in the Goldfish. A. Reverse-transcription PCR was performed to analyze the expression of Syntaxin 3A and 3B and beta actin in retina, brain and kidney. B. Real time PCR analysis of expression of syntaxin 3 A and 3B in goldfish retina. Data have been normalized to the value of syntaxin 3B. (Syntaxin 3A: 14.0 % ± 2.2 % (n=6); Syntaxin 3B: 100 % ± 9.5 % (n=6) (mean +/− s.e.m.)).
Fig. 3
Expression of syntaxin 3B in isolated retina bipolar cells. A. Single-cell Reverse-Transcription PCR was performed to confirm the presence of Syntaxin 3B in the Mb1 bipolar cell. Primers used for RT-PCR are marked on the right. Arrows mark the position of the specific PCR products. Input is labeled on top. Controls lane 2 and 4 show PCR reactions performed on mRNA from bipolar cells without reverse transcriptase and with added RNase A. Controls lanes 7 and 8 show PCR reactions performed on bath solution without cells. B. Representative pictures of an Mb 1 bipolar cell (left) and a horizontal cell (right) collected for single cell PCR. Inset is taken from an actual single cell PCR experiment and shows a bipolar cell being taken up into a collection pipette. Scale bars are 10µm.
Fig. 4
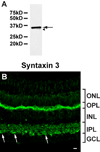
Syntaxin 3 is located in ribbon synapses in the goldfish retina. A. The purified syntaxin 3 antibody strongly reacted with a protein of the predicted size of syntaxin 3B in goldfish retina extract (arrow). A very weak, slower migrating band (labeled by an arrowhead) probably corresponds to a breakdown product. B. The syntaxin 3 antibody labels synaptic layers (inner and outer plexiform layers (IPL, OPL)) in a section of goldfish retina. Potential Mb1 bipolar terminals are marked by arrows. (ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer). Scale bar = 10 µm for all panels.
Fig. 5
Syntaxin 3 is located at the plasma-membrane of Mb1 bipolar terminals. Confocal image of a bipolar terminal labeled with antibodies against syntaxin 3 (A,C) and the synaptic vesicle marker SV2 (B,C). An optical section of 0.5 µm is depicted. The terminals of isolated Mb1 bipolar cells show strong labeling for Syntaxin 3B on the plasma-membrane (A,C). Arrow points to the synaptic terminal. Scale bar = 10 µm for all panels.
Fig. 6
A peptide derived from the SNARE domain of syntaxin 3B inhibits functional refilling in bipolar neurons. A. Isolated terminals were dialyzed with internal solution containing either the syntaxin 3B SNARE peptide (filled circles, n = 5) or a scrambled control peptide (open circles, n = 5.) Four 1 s depolarizing pulses (−60 to 0 mV) were given with an interpulse interval of 60 seconds. The change in membrane capacitance (ΔCm) measured for each pulse was normalized to the magnitude of the response to the first pulse. Data are expressed in mean ± s.e.m. p-values < 0.05 are marked with asterisks. B. The successive decrease in exocytosis is not due to decreased calcium influx. There was no significant difference in the mean peak amplitudes of the calcium current between cells dialyzed with the syntaxin 3B SNARE peptide (black bars, n = 5) and those dialyzed with the scrambled control (white bars, n = 5.)
Similar articles
- Phosphorylation of syntaxin 3B by CaMKII regulates the formation of t-SNARE complexes.
Liu X, Heidelberger R, Janz R. Liu X, et al. Mol Cell Neurosci. 2014 May;60:53-62. doi: 10.1016/j.mcn.2014.03.002. Epub 2014 Mar 27. Mol Cell Neurosci. 2014. PMID: 24680688 Free PMC article. - Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina.
Curtis LB, Doneske B, Liu X, Thaller C, McNew JA, Janz R. Curtis LB, et al. J Comp Neurol. 2008 Oct 10;510(5):550-9. doi: 10.1002/cne.21806. J Comp Neurol. 2008. PMID: 18683220 Free PMC article. - Conformational change of Syntaxin-3b in regulating SNARE complex assembly in the ribbon synapses.
Gething C, Ferrar J, Misra B, Howells G, Andrzejewski AL, Bowen ME, Choi UB. Gething C, et al. Sci Rep. 2022 Jun 3;12(1):9261. doi: 10.1038/s41598-022-09654-3. Sci Rep. 2022. PMID: 35661757 Free PMC article. - Synaptic release at mammalian bipolar cell terminals.
Wan QF, Heidelberger R. Wan QF, et al. Vis Neurosci. 2011 Jan;28(1):109-19. doi: 10.1017/S0952523810000453. Vis Neurosci. 2011. PMID: 21272392 Free PMC article. Review. - Syntaxin 3B: A SNARE Protein Required for Vision.
Dey H, Perez-Hurtado M, Heidelberger R. Dey H, et al. Int J Mol Sci. 2024 Oct 3;25(19):10665. doi: 10.3390/ijms251910665. Int J Mol Sci. 2024. PMID: 39408994 Free PMC article. Review.
Cited by
- Transmission at rod and cone ribbon synapses in the retina.
Thoreson WB. Thoreson WB. Pflugers Arch. 2021 Sep;473(9):1469-1491. doi: 10.1007/s00424-021-02548-9. Epub 2021 Mar 29. Pflugers Arch. 2021. PMID: 33779813 Free PMC article. Review. - SNARE complex in developmental psychiatry: neurotransmitter exocytosis and beyond.
Cupertino RB, Kappel DB, Bandeira CE, Schuch JB, da Silva BS, Müller D, Bau CH, Mota NR. Cupertino RB, et al. J Neural Transm (Vienna). 2016 Aug;123(8):867-83. doi: 10.1007/s00702-016-1514-9. Epub 2016 Feb 8. J Neural Transm (Vienna). 2016. PMID: 26856328 Review. - Presynaptic [Ca(2+)] and GCAPs: aspects on the structure and function of photoreceptor ribbon synapses.
Schmitz F. Schmitz F. Front Mol Neurosci. 2014 Feb 6;7:3. doi: 10.3389/fnmol.2014.00003. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24567702 Free PMC article. Review. - Syntaxin 1B, but not syntaxin 1A, is necessary for the regulation of synaptic vesicle exocytosis and of the readily releasable pool at central synapses.
Mishima T, Fujiwara T, Sanada M, Kofuji T, Kanai-Azuma M, Akagawa K. Mishima T, et al. PLoS One. 2014 Feb 28;9(2):e90004. doi: 10.1371/journal.pone.0090004. eCollection 2014. PLoS One. 2014. PMID: 24587181 Free PMC article. - Phosphorylation of the Retinal Ribbon Synapse Specific t-SNARE Protein Syntaxin3B Is Regulated by Light via a Ca2 +-Dependent Pathway.
Campbell JR, Li H, Wang Y, Kozhemyakin M, Hunt AJ Jr, Liu X, Janz R, Heidelberger R. Campbell JR, et al. Front Cell Neurosci. 2020 Oct 20;14:587072. doi: 10.3389/fncel.2020.587072. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192329 Free PMC article.
References
- Berntson AK, Morgans CW. Distribution of the presynaptic calcium sensors, synaptotagmin I/II and synaptotagmin III, in the goldfish and rodent retinas. J Vis. 2003;3:274–280. - PubMed
- Broadie K, Prokop A, Bellen HJ, O'Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. - PubMed
- Ciudad J, Cid E, Velasco A, Lara JM, Aijon J, Orfao A. Flow cytometry measurement of the DNA contents of G0/G1 diploid cells from three different teleost fish species. Cytometry. 2002;48:20–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY012128/EY/NEI NIH HHS/United States
- EY010608/EY/NEI NIH HHS/United States
- T32 EY007024/EY/NEI NIH HHS/United States
- EY016452/EY/NEI NIH HHS/United States
- RR022531/RR/NCRR NIH HHS/United States
- S10 RR022531/RR/NCRR NIH HHS/United States
- R29 EY012128/EY/NEI NIH HHS/United States
- R01 EY012128-12/EY/NEI NIH HHS/United States
- P30 EY010608/EY/NEI NIH HHS/United States
- EY012128/EY/NEI NIH HHS/United States
- R01 EY016452-04/EY/NEI NIH HHS/United States
- R01 EY016452/EY/NEI NIH HHS/United States
- T32 NS007467/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous