Regulation of photoreceptor gene expression by the retinal homeobox (Rx) gene product - PubMed (original) (raw)
Regulation of photoreceptor gene expression by the retinal homeobox (Rx) gene product
Yi Pan et al. Dev Biol. 2010.
Abstract
The retinal homeobox (Rx) gene product is essential for eye development. However little is known about its molecular function. It has been demonstrated that Rx binds to photoreceptor conserved element (PCE-1), a highly conserved element found in the promoter region of photoreceptor-specific genes such as rhodopsin and red cone opsin. We verify that Rx is co-expressed with rhodopsin and red cone opsin in maturing photoreceptors and demonstrate that Rx binds to the rhodopsin and red cone opsin promoters in vivo. We also find that Rx can cooperate with the Xenopus analogs of Crx and Nrl, otx5b and XLMaf (respectively), to activate a Xenopus opsin promoter-dependent reporter. Finally, we demonstrate that reduction of Rx expression in tadpoles results in decreases in expression of several PCE-1 containing photoreceptor genes, abnormal photoreceptor morphology, and impaired vision. Our data suggests that Rx, in combination with other transcription factors, is necessary for normal photoreceptor gene expression, maintenance, and function. This establishes a direct role for Rx in regulation of genes expressed in a differentiated cell type.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
Figure 1
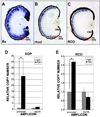
Rhodopsin and red cone opsin (RCO) are Rx targets. A – C. Rx is co-expressed with rhodopsin and RCO in photoreceptors. In situ hybridization on sections from paraffin-embedded st 41 tadpoles using probes for Rx (A), rhodopsin (B) or RCO (C). D, E. Chromatin immunoprecipitation (ChIP) results indicating that myc-tagged Rx (MT-Rx) can bind to the rhodopsin (D) and RCO (E) promoters in vivo. Results are presented as the CT of each sample normalized to the CT of a “no antibody” control. * - p < 0.003 (XOP), p < 0.002 (RCO).
Figure 2
Rx and RxL can cooperate with other factors to activate the Xenopus opsin promoter (XOP). Luciferase assay performed using lysates from embryos co-injected with XOP-Luc reporter plasmid and Rx, Rx-L, otx5b, and/or XLmaf RNAs as shown. A. Rx activates XOP-Luc in a dose-dependent manner. B. Rx cooperates with otx5b and XLmaf to activate the XOP-Luc reporter. C. Rx-L cooperates with otx5b and XLmaf to activate the XOP-Luc reporter.
Figure 3
Generation of Rx knockdown embryos. A. A portion of X. laevis Rx1A was selected as a target for development of an shRNA. Alignment of the shRNA target sequence with corresponding regions of X. laevis Rx2A and mouse Rx. B. Tadpoles transgenic for the Rx shRNA plasmid appeared normal. Bright light or UV-light views of transgenic tadpoles at st 41. C. Retinas from Rx shRNA transgenic tadpoles appeared histologically normal. Hematoxylin and eosin stained sections of paraffin-embedded wild type (left panel) or Rx shRNA transgenic tadpole. D. Rx shRNA transgenic tadpoles have reduced levels of both Rx1A and Rx2A as determined by quantitative RT-PCR (qRT-PCR) performed using total RNA purified from isolated tadpole heads (st 41). E. Rx1A is expression is reduced by in situ hybridization performed using 8 µM sections of paraffin-embedded tadpoles at st 38, 41, and 45 (from left to right). The reduction in Rx expression appears to increase as development progresses.
Figure 4
Exogenous Argonaute2 (Ago2) exacerbates the effects of shRNA-mediated Rx knockdown. A. Exogenous Ago2 exacerbates the Rx shRNA knockdown phenotype. Embryos were generated by intra-cytosolic sperm injection (ICSI) with Rx shRNA transgene and injected with RNA encoding X. laevis Ago2 RNA. Embryos were co-injected with dsRed Express RNA as a lineage tracer. Embryos were photographed under white light (i, ii, iii), red fluorescence to visualize dsRed lineage tracer (i’, ii’, iii’), or blue fluorescence to visualize Rx shRNA trasngene (i”, ii”, iii”). Embryos receiving only the Rx shRNA (panels i – iii) or Ago2 RNA (panels i” – iii”) have apparently normal eyes while embryos transgenic for the Rx shRNA and receiving Ago2 RNA in the developing eye exhibited abnormally developed eyes (panels i’ – iii’). B. Exogenous Ago2 exacerbates the effects of Rx shRNA on Rx expression. Wholemount in situ hybridization using a Rx antisense riboprobe and embryos receiving either the Rx shRNA transgene (ii), Ago2 RNA (iii), or both (iv). Control embryos (i) received neither. These results are presented in graph form in C.
Figure 5
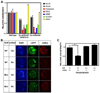
Photoreceptor gene expression is reduced by Rx knockdown. A. Expression of the photoreceptor genes rhodopsin, red cone opsin, IRBP, and arrestin are reduced in Rx shRNA transgenic tadpoles by qRT-PCR, as are Rx1A and Rx2A. Expression of silver is not reduced in Rx shRNA transgenic embryos by qRT-PCR. B. Rx knockdown results in PCE-1 site-dependent reduction in rhodopsin promoter activity. Co-transgenic tadpoles were generated using a Rx or control shRNA transgene and XOP-dsRed containing a wild type or mutated PCE-1 site, as indicated on the left side of the figure. Left column: retinal cell nuclei visualized using DAPI. Middle column: shRNA transgene visualized by the coral GFP (cGFP) expression cassette. Right column: XOP-dsRed trasngene expression. C. Quantification of results shown in B as described in Methods. Rx shRNA results in reduction of XOP-dsRed as compared to control (reversed sequence) shRNA. Introduction of the Rx shRNA transgene does not result in decreased XOP-dsRed expression level when the PCE-1 site is mutated. * - p < 0.025. Abbreviations: WT – wild type; Ctl – control.
Figure 6
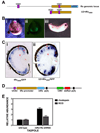
Knockdown of photopigment gene expression can be rescued by expression of mouse Rx. A. Top: Schematic diagram of the X. tropicalis Rx genomic locus. Shown are: two of the three ultra-conserved elements (UCEs) (UCE2 and UCE3, purple), 3 kb promoter (yellow), first coding exon (blue). Bottom: Schematic diagram of Rx regulatory region construct UCE2+tRx3000, containing the 3 kb Rx promoter and UCE2. B. Expression of tRx3000/GFP transgene in tailbud embryos. i: white light image of st 20 neural tube stage embryo; ii: fluorescent image of the same embryo shown in (i); iii: wholemount in situ hybridization of tailbud stage (st 28) tRx3000/GFP transgenic embryo using an antisense riboprobe to GFP. C. In situ hybridization of sections of paraffin-embedded st 41 tadpoles using an antisense riboprobe to GFP. i: The tRx3000/GFP transgene is expressed in the photoreceptor layer, in the INL, and the CMZ. It is not expressed in the distal portion of the CMZ where retinal stem cells are found. ii: Addition of UCE2 to the tRx3000/GFP transgene drives expression throughout the CMZ. D. Schematic diagram of rescue construct. The construct includes the mRx coding region driven by UCE2+tRx3000 and a dsRed expression cassette driven by the CMV promoter for selection of transgenic embryos. E. Expression of photopigment genes rhodopsin and red cone opsin is not reduced in embryos transgenic for both Rx shRNA and the mRx by qRT-PCR.
Figure 7
Specific degeneration of photoreceptors in visually impaired Rx shRNA tadpoles. A – C. Hematoxylin and eosin staining of sections prepared from paraffin-embedded st 50 tadpoles. Black arrows indicate nuclei in the outer nuclear layer (ONL). Red arrow indicates a gap in the ONL. D – F. Immunohistochemical staining of sections from paraffin-embedded tadpoles using an antibody raised against rhodopsin (RetP1). Black arrows indicate RetP1-positive cells in the photoreceptor layer. Red arrow indicates a gap in RetP1 staining in the photoreceptor layer. G – I. Staining of sections prepared from paraffin-embedded tadpoles using peanut agglutinin (PNA). Black arrows indicate PNA-positive cells in the photoreceptor layer. Red arrow indicates a gap in PNA staining in the photoreceptor layer. Wild type (A, D, G), Rx shRNA transgenic (B, E, H), or Rx shRNA + mRx rescue construct cotransgenic (C, F, I) tadpoles were raised to st 50 and tested for visual function (Table 1). Rx shRNA transgenic tadpoles with impaired visual function exhibited abnormal photoreceptor histology, including missing nuclei (red arrow) compared to a nontransgenic control (A, B). Photoreceptor histology was normal in a tadpole transgenic for both Rx shRNA and mRx (C). Rod and cone photoreceptors of Rx shRNA tadpoles exhibited reduced staining with rhodopsin and PNA (red arrows in E and H) compared to nontransgenic controls (D, E, G, H). Rhodopsin and PNA staining appeared normal in tadpoles transgenic for both the Rx shRNA and mRx (F, I).
Similar articles
- Regulation of photoreceptor gene transcription via a highly conserved transcriptional regulatory element by vsx gene products.
Pan Y, Comiskey DF, Kelly LE, Chandler DS, El-Hodiri HM. Pan Y, et al. Mol Vis. 2016 Dec 14;22:1421-1428. eCollection 2016. Mol Vis. 2016. PMID: 28003732 Free PMC article. - Derivation of human differential photoreceptor-like cells from the iris by defined combinations of CRX, RX and NEUROD.
Seko Y, Azuma N, Kaneda M, Nakatani K, Miyagawa Y, Noshiro Y, Kurokawa R, Okano H, Umezawa A. Seko Y, et al. PLoS One. 2012;7(4):e35611. doi: 10.1371/journal.pone.0035611. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558175 Free PMC article. - The Rx-like homeobox gene (Rx-L) is necessary for normal photoreceptor development.
Pan Y, Nekkalapudi S, Kelly LE, El-Hodiri HM. Pan Y, et al. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4245-53. doi: 10.1167/iovs.06-0167. Invest Ophthalmol Vis Sci. 2006. PMID: 17003412 Free PMC article. - The role of Xenopus Rx-L in photoreceptor cell determination.
Wu HY, Perron M, Hollemann T. Wu HY, et al. Dev Biol. 2009 Mar 15;327(2):352-65. doi: 10.1016/j.ydbio.2008.12.017. Epub 2008 Dec 25. Dev Biol. 2009. PMID: 19135436 - Regulation of vertebrate eye development by Rx genes.
Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers PH, Jamrich M. Bailey TJ, et al. Int J Dev Biol. 2004;48(8-9):761-70. doi: 10.1387/ijdb.041878tb. Int J Dev Biol. 2004. PMID: 15558469 Review.
Cited by
- Cooperative activation of Xenopus rhodopsin transcription by paired-like transcription factors.
Reks SE, McIlvain V, Zhuo X, Knox BE. Reks SE, et al. BMC Mol Biol. 2014 Feb 6;15:4. doi: 10.1186/1471-2199-15-4. BMC Mol Biol. 2014. PMID: 24499263 Free PMC article. - Regulation of photoreceptor gene transcription via a highly conserved transcriptional regulatory element by vsx gene products.
Pan Y, Comiskey DF, Kelly LE, Chandler DS, El-Hodiri HM. Pan Y, et al. Mol Vis. 2016 Dec 14;22:1421-1428. eCollection 2016. Mol Vis. 2016. PMID: 28003732 Free PMC article. - Regulation of retinal homeobox gene transcription by cooperative activity among cis-elements.
Martinez-de Luna RI, Moose HE, Kelly LE, Nekkalapudi S, El-Hodiri HM. Martinez-de Luna RI, et al. Gene. 2010 Nov 1;467(1-2):13-24. doi: 10.1016/j.gene.2010.07.005. Epub 2010 Jul 11. Gene. 2010. PMID: 20627122 Free PMC article. - Lxr regulates lipid metabolic and visual perception pathways during zebrafish development.
Pinto CL, Kalasekar SM, McCollum CW, Riu A, Jonsson P, Lopez J, Swindell EC, Bouhlatouf A, Balaguer P, Bondesson M, Gustafsson JÅ. Pinto CL, et al. Mol Cell Endocrinol. 2016 Jan 5;419:29-43. doi: 10.1016/j.mce.2015.09.030. Epub 2015 Sep 30. Mol Cell Endocrinol. 2016. PMID: 26427652 Free PMC article.
References
- Andreazzoli M, Gestri G, Angeloni D, Menna E, Barsacchi G. Role of Xrx1 in Xenopus eye and anterior brain development. Development. 1999;126:2451–2460. - PubMed
- Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers PH, Jamrich M. Regulation of vertebrate eye development by Rx genes. Int J Dev Biol. 2004;48:761–770. - PubMed
- Batni S, Scalzetti L, Moody SA, Knox BE. Characterization of the Xenopus rhodopsin gene. J Biol Chem. 1996;271:3179–3186. - PubMed
- Boatright JH, Borst DE, Peoples JW, Bruno J, Edwards CL, Si JS, Nickerson JM. A major cis activator of the IRBP gene contains CRX-binding and Ret-1/PCE-I elements. Mol Vis. 1997;3:15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials