The indispensable N-terminal half of eIF3j/HCR1 cooperates with its structurally conserved binding partner eIF3b/PRT1-RRM and with eIF1A in stringent AUG selection - PubMed (original) (raw)
The indispensable N-terminal half of eIF3j/HCR1 cooperates with its structurally conserved binding partner eIF3b/PRT1-RRM and with eIF1A in stringent AUG selection
Latifa Elantak et al. J Mol Biol. 2010.
Abstract
Despite recent progress in our understanding of the numerous functions of individual subunits of eukaryotic translation initiation factor (eIF) 3, little is known on the molecular level. Using NMR spectroscopy, we determined the first solution structure of an interaction between eIF3 subunits. We revealed that a conserved tryptophan residue in the human eIF3j N-terminal acidic motif (NTA) is held in the helix alpha1 and loop 5 hydrophobic pocket of the human eIF3b RNA recognition motif (RRM). Mutating the corresponding "pocket" residues in its yeast orthologue reduces cellular growth rate, eliminates eIF3j/HCR1 association with eIF3b/PRT1 in vitro and in vivo, affects 40S occupancy of eIF3, and produces a leaky scanning defect indicative of a deregulation of the AUG selection process. Unexpectedly, we found that the N-terminal half of eIF3j/HCR1 containing the NTA is indispensable and sufficient for wild-type growth of yeast cells. Furthermore, we demonstrate that deletion of either j/HCR1 or its N-terminal half only, or mutation of the key tryptophan residues results in the severe leaky scanning phenotype partially suppressible by overexpressed eIF1A, which is thought to stabilize properly formed preinitiation complexes at the correct start codon. These findings indicate that eIF3j/HCR1 remains associated with the scanning preinitiation complexes and does not dissociate from the small ribosomal subunit upon mRNA recruitment, as previously believed. Finally, we provide further support for earlier mapping of the ribosomal binding site for human eIF3j by identifying specific interactions of eIF3j/HCR1 with small ribosomal proteins RPS2 and RPS23 located in the vicinity of the mRNA entry channel. Taken together, we propose that eIF3j/HCR1 closely cooperates with the eIF3b/PRT1 RRM and eIF1A on the ribosome to ensure proper formation of the scanning-arrested conformation required for stringent AUG recognition.
(c) 2009 Elsevier Inc. All rights reserved.
Figures
Figure 1
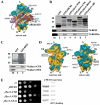
The CTD of j/HCR1 interacts with RPS2 and 23a situated near the 40S mRNA-entry channel but it is dispensable for efficient translation in yeast as opposed to its NTD. (A) Hypothetical location of eIF3 on the solvent side of the S. cerevisiae 40S subunit based on Cryo-EM reconstruction (adapted from 14). Protrusion of the CTD of eIF3j into the mRNA entry channel based on is symbolized by a green arrow. The blue lines represent mRNA. (B and C) The j/hcr1-CTD interacts with RPS2 and RPS23a via its KERR motif. (B) Full-length j/HCR1 (lane 3), its N-terminal (lane 4) or C-terminal (lane 5) halves, various mutants (lanes 6 and 7) defined in Fig. 6B fused to GST, and GST alone (lane 2), were tested for binding against 35S-labeled wt RPS2 and RPS23; the 10% of input amounts added to each reaction is shown in lane 1 (In). (C) RPS2 fused to GST (lane 3) and GST alone (lane 2) were tested for binding to 35S-labeled j/hcr1-CTD and NTD essentially the same as in 1B. (D) Cryo-EM reconstruction of the S. cerevisiae 40S subunit (adapted from 28). The 40S subunit is shown from the interface (left) or solvent (right) sides, with RNA segments in yellow and proteins in green. The mRNA entry and exit channels are designated by * and X, respectively. (E) The j/hcr1-NTD is required for wt growth dependent on its intact NTA motif. Transformants of H428 (j/hcr1Δ) bearing empty vector; YEp-j/HCR1-DS; YEp-j/hcr1-CTD; YEp-j/hcr1-NTD; and YEp-j/hcr1-NTA1; respectively, were spotted in five serial 10-fold dilutions on SD medium and incubated at 30°C for 1.5 days. The far-right columns contain results of Western analysis of WCEs from the very same strains using anti-j/HCR1 (j/HCR1 expression) and anti-GCD11 (eIF2γ loading) antibodies, respectively.
Figure 2
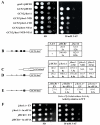
Both the NTD and CTD of j/HCR1 retain intrinsic 40S-binding affinity. (A-D) Transformants of strain H428 (j/hcr1Δ) bearing YEp-j/HCR1-DS; YEp-j/hcr1-NTD; YEp-j/hcr1-CTD; and YEp-j/hcr1-NTD-NTA1, respectively, were grown in SD medium at 30°C to an OD600of ~ 1.5 and cross-linked with 2% HCHO prior to harvesting. WCE were prepared and subsequently separated on a 7.5%-30% sucrose gradient by centrifugation at 41,000 rpm for 5 h. The 40S fractions were pooled, resolved on a second gradient, and subjected to Western analysis. First two fractions were combined (top). Proportions of the 40S-bound j/HCR1 proteins relative to the amount of 40S subunits were calculated using NIH ImageJ from two independent experiments. The resulting values obtained with the wt strain were set to 100% and those obtained with mutant strains were expressed as percentages of the wt (SDs are given). (E-F) Genetic evidence that j/HCR1 with intact NTA (or only its NTD) stimulates 40S-binding by eIF3. (E) Overexpression of the TC partially suppresses the Slg− of j/hcr1Δ and_j/hcr1-NTA1_ mutants. H416 (j/HCR1; rows 1 and 2), H428 (j/hcr1Δ YEplac181; rows 3 and 4), and SY73 (j/hcr1Δ YEp-j/hcr1-NTA1; rows 5 and 6), respectively, were transformed with either empty vector (rows 1, 3, and 5) or the TC overexpressing vector (rows 2, 4, and 6) and the resulting transformants were spotted in four serial 10-fold dilutions on SD medium and incubated at 30°C for 2 days. (F) j/HCR1 with intact NTA (or only its NTD) partially suppresses the Ts− phenotype of_b/prt1-rnp1_. Transformants of the strain H3674 (b/prt1-rnp1) bearing empty vector; YEp-j/HCR1-DS; YEp-j/hcr1-NTD; YEp-j/hcr1-CTD; and YEp-j/hcr1-NTA1, respectively, were spotted in four serial 10-fold dilutions on SD medium and incubated at 33°C for 3 days.
Figure. 3
Genetic evidence that the deletion of the j/hcr1-NTD (or the NTA1 mutation) prevents derepression of GCN4 translation during starvation as a result of leaky scanning partially suppressible by high copy eIF1A. (A)j/hcr1Δ imparts a Gcn−phenotype implicating j/HCR1 in GCN4 translational control. H418 (gcn2Δ j/HCR1; row 1) and the H428 (GCN2 j/hcr1Δ) transformants bearing YEp-j/HCR1 (row 2); YEplac181 (row 3); YEp-j/hcr1-NTD (row 4); YEp-j/hcr1-CTD (row 5); YEp-j/hcr1-NTA1 (row 6); and YEp-j/hcr1-NTD-NTA1 (row 7), respectively, were spotted in four serial 10-fold dilutions on SD (left panel) or SD containing 30 mM 3-AT (right panel) and then incubated at 30°C for 2 and 3 days, respectively. (B) j/hcr1Δ prevents full derepression of GCN4-lacZ expression upon starvation. Isogenic H416 (GCN2 j/HCR1) and H428 were transformed with p180, grown in minimal media for 6 hours and the β-galactosidase activities were measured in the WCEs and expressed in units of nmol of o-nitrophenyl-b-D-galactopyranoside hydrolyzed per min per mg of protein. To induce GCN4-lacZ expression, transformants grown at the minimal media for 2 hrs were treated with 10 mM 3-AT for 6 hrs. The table gives mean values and standard deviations obtained from at least 6 independent measurements with three independent transformants, and activity in j/hcr1Δ relative to wt, respectively. (C - D) Deletion of j/hcr1Δ or its NTD only dramatically increases leaky scanning. H428 transformants bearing YEp-j/HCR1; YEplac181; YEp-j/hcr1-CTD; YEp-j/hcr1-NTD; and YEp-j/hcr1-NTA1, respectively, were transformed with pM226 (C) and plig102-3 (D), respectively, and analyzed as in (B), except that they were not treated with 3-AT. (E) Overexpression of eIF1A partially suppresses the leaky scanning defect of_j/hcr1Δ_. Strains H416 and H428 transformed with empty vector and YEpTIF11 (eIF1A), respectively, were transformed with plig102-3 and analyzed as in (D). (F) Overexpression of eIF1A partially suppresses the Slg− and Gcn− phenotypes of j/hcr1Δ. Strains H428 and H416 transformed with empty vector and YEpTIF11, respectively, were spotted in four serial 10-fold dilutions on SD or SD + 30 mM 3-AT and incubated at 30°C for 2 or 4 days.
Figure. 4
Structure of the heIF3b-RRM - heIF3j complex. (A) NMR ensemble of heIF3b-RRM - heIF3j peptide complex. The 10 lowest-energy structures between heIF3b-RRM179-274 (in grey and green) and heIF3j45-55 (in yellow) are shown. The structures were fit using the backbone atoms C’, Cα, and N of residues 184-264 of heIF3b-RRM and residues 45-55 of heIF3j. (B) Ribbon model for the lowest-energy conformer of the heIF3b-RRM (in grey and green)/heIF3j (in yellow) complex. Secondary structure elements of heIF3b-RRM are labeled. (C) Surface representation of the contacts between heIF3j peptide and heIF3b-RRM. Green and blue surfaces indicate hydrophobic and basic (labeled) heIF3b-RRM residues, respectively. heIF3j peptide is shown as a ribbon ball-and-stick representation, and most of its residues are numbered with primed numbers. The lowest-energy structure of heIF3b-RRM bound to heIF3j peptide is shown. (D) Close-up view of the hydrophobic pocket binding the heIF3j peptide. The heIF3b-RRM is displayed as grayish semitransparent solvent-accessible surface with labeled hydrophobic side chains in green shown below the surface. These residues form the walls of the hydrophobic pocket in which the aromatic ring of W52′ of the heIF3j peptide in yellow is inserted (residues 51-53 only). (E) Comparison of NMR structures of free and heIF3j-bound heIF3b-RRM. The two structures are represented as ribbon models with helices α1 and α2 and loop L5 colored in green for heIF3j-bound and in blue for free heIF3b-RRM. The side chains of Y253 and I210 are shown in stick representation using the same coloring scheme to highlight closer contacts participating to a more compact conformation of heIF3b-RRM when bound to heIF3j.
Figure. 5
Mutational analysis of the heIF3b-RRM - heIF3j-NTA interaction. (A) Isothermal calorimetric titration of wt and mutant heIF3j with heIF3b-RRM. The panel shows the fitted binding isotherms. The data points were obtained by integration of heat signals plotted against the molar ratio of heIF3b-RRM to wt or mutant heIF3j in the reaction cell. The solid line represents a calculated curve using the best fit parameters obtained by a nonlinear least squares fit. The heIF3j construct used for each experiment is indicated in the panel. (B) Histidine pull-down assays using His6-tagged wt or mutant heIF3b-RRM and untagged heIF3j. SDS-PAGE analysis of the input and eluted fractions (I and E) from the pull-down experiments where the untagged heIF3j subunit was used with wt or mutant (F261A, I210A, Y253A) heIF3b-RRM as well as alone (Resin only) as a control. (C) Quantification of heIF3j fraction bound to heIF3b-RRM by analyzing the band intensity of the eluted fraction compared to the same band in the input fraction. Error bars represent the standard deviation between two individual experiments.
Figure. 6
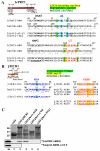
Molecular determinants of the eIF3j–eIF3b-RRM interaction are evolutionary conserved. (A) Schematic of b/PRT1 showing the position of the RNA recognition motif (rrm). Arrows delimit minimal binding domains for the indicated proteins. Positions of both RNPs (black), helix α1 (blue) and loop L5 (red) are indicated above the sequences aligned using the GCG Analysis Program. Residues corresponding to the heIF3j binding surface are highlighted in yellow, residues forming the hydrophobic pocket in green. Underlined are b/PRT1 residues that were subjected to site-directed mutagenesis in this or previous studies . (B) Same as in (A) except that the schematic of j/HCR1 is shown with locations of the N-terminal acid motif (nta), the C-terminal KERR motif (kerr), and the C-terminal truncation (Δ80). Sequences surrounding the NTA and KERR motifs of yeast and human eIF3j or eIF3a, respectively, are indicated. Underlined are j/HCR1 resides that were subjected to site-directed mutagenesis in this or previous studies . The human Trp52 and the corresponding yeast Trp37 are highlighted in green; the key residues of the KERR motif in yellow. (C) Full-length j/HCR1 (lane 3), its N-terminal (lane 4) or C-terminal (lane 5) halves, the NTA1 mutant (lane 6) fused to GST, and GST alone (lane 2), were tested for binding to 35S-labeled wt b/prt1-RRM [1-136] and b/prt1-rrm-α1+L5; the 10% of input amounts added to each reaction is shown in lane 1 (In).
Figure. 7
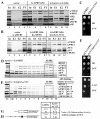
Destroying the hydrophobic pocket of the RRM of b/PRT1 prevents j/HCR1-association with eIF3 in vivo, reduces eIF3-binding to 40S subunits, and increases leaky scanning. (A – B) The NTA of j/HCR1 and α1 and L5 of b/PRT1-RRM are critically required for j/HCR1 association with the MFC in vivo. (A) WCEs were prepared from H425 (b/prt1Δ) bearing untagged b/PRT1/PRT1 (lanes 1 to 4), and H425 transformants with pRS-b/PRT1-His (lanes 5 to 8) and pRS-b/prt1-L5+α1-His (lanes 9 to 12) from which the untagged b/PRT1 was evicted on 5-FOA, respectively, were incubated with Ni2+ silica resin, and the bound proteins were eluted and subjected to Western blot analysis with antibodies indicated on the right-handed side of individual strips. Lanes 1, 5 and 9 contained 5% of the input WCEs (In); lanes 2, 6, and 10 contained 30% of fractions eluted from the resin (E1); lanes 3, 7, and 11 contained 60% of the same fractions (E2); and lanes 4, 8, and 12 contained 5% of the flow through (FT). (B) WCEs prepared from double transformants of H428 (j/hcr1Δ) bearing pRS315 and YCp-j/HCR1-DS-U (lanes 1 to 4); pRS-b/PRT1-His and YEp-j/HCR1-DS-U (lanes 5 to 8), or pRS-b/PRT1-His and YEp-j/hcr1-NTA1-U (lanes 9 to 12), respectively, were analyzed as in (A). (C – E) Mutating the hydrophobic pocket of the RRM of b/PRT1 results in the Slg- phenotype and strongly affects 40S-association of eIF3. (C) H425 transformants as in (A) were spotted in four serial 10-fold dilutions on SD medium and incubated at 37°C for 2 days. (D - E) H425 transformants as in (A) were grown in SD medium at 37°C to an OD600 of ~ 1.5 and analyzed as in Figure 2A-D except that the resedimentation protocol was not applied. Mean proportions of the total proteins found in fractions 10-11 were calculated using NIH ImageJ from two independent experiments. The resulting values obtained with the indicated eIFs with the wt strain were set to 100% and those obtained with the mutant strain were expressed as percentages of the wt (SDs are given). (F - H) The_b/prt1-α1_+L5 mutation impairs derepression of GCN4 translation during starvation as a result of leaky scanning. (F) b/prt1-α1+L5 imparts the Gcn− phenotype. YAH06 (_GCN2 b/prt1_Δ) transformants carrying pRS-b/PRT1-His and pRS-b/prt1-L5+α1-His, respectively, were spotted in four serial 10-fold dilutions on SD (upper panel) or SD containing 30 mM 3-AT (bottom) and then incubated at 34°C for 2 and 3 days, respectively. (G and H)b/prt1-α1+L5 strongly increases leaky scanning. YAH06 transformants as in (F) were transformed with pM226 (G) and plig102-3 (H), respectively, and analyzed as in Fig. 3C and D.
Figure 8
eIF3j/HCR1 co-operates with eIF3b/PRT1 and eIF1A to ensure stringent selection of the AUG start codon. (A) In the absence of eIFs, eIF3j/HCR1 occupies the mRNA entry channel to at least partially block mRNA recruitment. (B) Recruitment of TC and eIF3 that interacts with the NTA of eIF3j/HCR1 via the RRM of eIF3b/PRT1 clears the mRNA entry channel so that the ribosome can adopt the open, scanning-conducive conformation for mRNA recruitment. (C) Upon AUG recognition, eIF3j/HCR1 in cooperation with the eIF3b/PRT1-RRM functionally interacts with eIF1A to stimulate prompt switching to the closed, scanning-arrested conformation. Black thick lines represent direct interactions; dotted line with arrowheads indicates functional interaction between eIF3j/HCR1 and eIF1A.
Similar articles
- Interaction of the RNP1 motif in PRT1 with HCR1 promotes 40S binding of eukaryotic initiation factor 3 in yeast.
Nielsen KH, Valásek L, Sykes C, Jivotovskaya A, Hinnebusch AG. Nielsen KH, et al. Mol Cell Biol. 2006 Apr;26(8):2984-98. doi: 10.1128/MCB.26.8.2984-2998.2006. Mol Cell Biol. 2006. PMID: 16581774 Free PMC article. - The C-terminal region of eukaryotic translation initiation factor 3a (eIF3a) promotes mRNA recruitment, scanning, and, together with eIF3j and the eIF3b RNA recognition motif, selection of AUG start codons.
Chiu WL, Wagner S, Herrmannová A, Burela L, Zhang F, Saini AK, Valásek L, Hinnebusch AG. Chiu WL, et al. Mol Cell Biol. 2010 Sep;30(18):4415-34. doi: 10.1128/MCB.00280-10. Epub 2010 Jun 28. Mol Cell Biol. 2010. PMID: 20584985 Free PMC article. - Crystal structure of the RNA recognition motif of yeast translation initiation factor eIF3b reveals differences to human eIF3b.
Khoshnevis S, Neumann P, Ficner R. Khoshnevis S, et al. PLoS One. 2010 Sep 16;5(9):e12784. doi: 10.1371/journal.pone.0012784. PLoS One. 2010. PMID: 20862284 Free PMC article. - In vivo deletion analysis of the architecture of a multiprotein complex of translation initiation factors.
Nielsen KH, Valásek L. Nielsen KH, et al. Methods Enzymol. 2007;431:15-32. doi: 10.1016/S0076-6879(07)31002-1. Methods Enzymol. 2007. PMID: 17923228 Review. - Conservation and diversity in the structure of translation initiation factor EIF3 from humans and yeast.
Hershey JW, Asano K, Naranda T, Vornlocher HP, Hanachi P, Merrick WC. Hershey JW, et al. Biochimie. 1996;78(11-12):903-7. doi: 10.1016/s0300-9084(97)86711-9. Biochimie. 1996. PMID: 9150866 Review.
Cited by
- The eIF3c/NIP1 PCI domain interacts with RNA and RACK1/ASC1 and promotes assembly of translation preinitiation complexes.
Kouba T, Rutkai E, Karásková M, Valášek L. Kouba T, et al. Nucleic Acids Res. 2012 Mar;40(6):2683-99. doi: 10.1093/nar/gkr1083. Epub 2011 Nov 28. Nucleic Acids Res. 2012. PMID: 22123745 Free PMC article. - RNA interference-mediated silencing of eukaryotic translation initiation factor 3, subunit B (EIF3B) gene expression inhibits proliferation of colon cancer cells.
Wang Z, Chen J, Sun J, Cui Z, Wu H. Wang Z, et al. World J Surg Oncol. 2012 Jun 26;10:119. doi: 10.1186/1477-7819-10-119. World J Surg Oncol. 2012. PMID: 22734884 Free PMC article. - Heterogeneity and specialized functions of translation machinery: from genes to organisms.
Genuth NR, Barna M. Genuth NR, et al. Nat Rev Genet. 2018 Jul;19(7):431-452. doi: 10.1038/s41576-018-0008-z. Nat Rev Genet. 2018. PMID: 29725087 Free PMC article. Review. - Reprogramming mRNA Expression in Response to Defect in RNA Polymerase III Assembly in the Yeast Saccharomyces cerevisiae.
Rudzińska I, Cieśla M, Turowski TW, Armatowska A, Leśniewska E, Boguta M. Rudzińska I, et al. Int J Mol Sci. 2021 Jul 7;22(14):7298. doi: 10.3390/ijms22147298. Int J Mol Sci. 2021. PMID: 34298922 Free PMC article. - Examining weak protein-protein interactions in start codon recognition via NMR spectroscopy.
Luna RE, Akabayov SR, Ziarek JJ, Wagner G. Luna RE, et al. FEBS J. 2014 Apr;281(8):1965-73. doi: 10.1111/febs.12667. Epub 2014 Jan 2. FEBS J. 2014. PMID: 24393460 Free PMC article. Review.
References
- Hinnebusch AG, Dever TE, Asano KA. Mechanism of translation initiation in the yeast Saccharomyces cerevisiae. In: Sonenberg N, Mathews M, Hershey JWB, editors. Translational Control in biology and medicine. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2007. pp. 225–268.
- Pestova TV, Lorsch JR, Hellen CUT. The mechanism of translation initiation in eukaryotes. In: Sonenberg N, Mathews M, Hershey JWB, editors. Translational Control in biology and medicine. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2007. pp. 87–128.
- Passmore LA, Schmeing TM, Maag D, Applefield DJ, Acker MG, Algire MA, Lorsch JR, Ramakrishnan V. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol Cell. 2007;26:41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 TW007271-01/TW/FIC NIH HHS/United States
- 076456/WT_/Wellcome Trust/United Kingdom
- R01 TW007271/TW/FIC NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- 076456/Z/05/Z/WT_/Wellcome Trust/United Kingdom
- MC_U105170649/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous