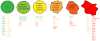Eukaryotic stress granules: the ins and outs of translation - PubMed (original) (raw)
Review
Eukaryotic stress granules: the ins and outs of translation
J Ross Buchan et al. Mol Cell. 2009.
Abstract
The stress response in eukaryotic cells often inhibits translation initiation and leads to the formation of cytoplasmic RNA-protein complexes referred to as stress granules. Stress granules contain nontranslating mRNAs, translation initiation components, and many additional proteins affecting mRNA function. Stress granules have been proposed to affect mRNA translation and stability and have been linked to apoptosis and nuclear processes. Stress granules also interact with P-bodies, another cytoplasmic RNP granule containing nontranslating mRNA, translation repressors, and some mRNA degradation machinery. Together, stress granules and P-bodies reveal a dynamic cycle of distinct biochemical and compartmentalized mRNPs in the cytosol, with implications for the control of mRNA function.
2009 Elsevier Inc.
Figures
Figure 1. A continuum of mRNP granules
Select examples of mRNP granules with compositional similarities to both stress granules and P-bodies: C.elegans blastomere germ granules, Gallo et al, 2008; C.elegans arrested ovulation oocyte foci, Jud et al, 2008; Drosophila neuronal transport granules, Barbee et al, 2006; Dendritic P-body, Cougot et al, 2008. Components observed solely in stress granules are highlighted in red, those solely in P-bodies in green, and those seen in both foci are highlighted in yellow. Lists are not necessarily exhaustive, and with specific experimental manipulation, some P-body/stress granule ‘distinct’ components have been observed in both structures.
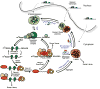
Figure 2. Model integrating Stress Granules, and P-bodies, into an mRNP cycle
A speculative model for mRNP transitions, particularly during stress. Dashed arrows indicate possible destination of exported nascent transcripts. Wavy purple lines represent microtubules, and their possible contribution of dynein/kinesin-mediated motorized transport to granule aggregation, and/or movement of mRNPs between different mRNP states.
Similar articles
- Multicolour single-molecule tracking of mRNA interactions with RNP granules.
Moon SL, Morisaki T, Khong A, Lyon K, Parker R, Stasevich TJ. Moon SL, et al. Nat Cell Biol. 2019 Feb;21(2):162-168. doi: 10.1038/s41556-018-0263-4. Epub 2019 Jan 21. Nat Cell Biol. 2019. PMID: 30664789 Free PMC article. - Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs.
Balagopal V, Parker R. Balagopal V, et al. Curr Opin Cell Biol. 2009 Jun;21(3):403-8. doi: 10.1016/j.ceb.2009.03.005. Epub 2009 Apr 23. Curr Opin Cell Biol. 2009. PMID: 19394210 Free PMC article. Review. - Principles of Stress Granules Revealed by Imaging Approaches.
Van Treeck B, Parker R. Van Treeck B, et al. Cold Spring Harb Perspect Biol. 2019 Feb 1;11(2):a033068. doi: 10.1101/cshperspect.a033068. Cold Spring Harb Perspect Biol. 2019. PMID: 30709880 Free PMC article. Review. - Analyzing P-bodies and stress granules in Saccharomyces cerevisiae.
Buchan JR, Nissan T, Parker R. Buchan JR, et al. Methods Enzymol. 2010;470:619-40. doi: 10.1016/S0076-6879(10)70025-2. Epub 2010 Mar 1. Methods Enzymol. 2010. PMID: 20946828 - P-bodies and stress granules: possible roles in the control of translation and mRNA degradation.
Decker CJ, Parker R. Decker CJ, et al. Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a012286. doi: 10.1101/cshperspect.a012286. Cold Spring Harb Perspect Biol. 2012. PMID: 22763747 Free PMC article. Review.
Cited by
- Yeast Gis2 and its human ortholog CNBP are novel components of stress-induced RNP granules.
Rojas M, Farr GW, Fernandez CF, Lauden L, McCormack JC, Wolin SL. Rojas M, et al. PLoS One. 2012;7(12):e52824. doi: 10.1371/journal.pone.0052824. Epub 2012 Dec 21. PLoS One. 2012. PMID: 23285195 Free PMC article. - Long-term memory consolidation: The role of RNA-binding proteins with prion-like domains.
Sudhakaran IP, Ramaswami M. Sudhakaran IP, et al. RNA Biol. 2017 May 4;14(5):568-586. doi: 10.1080/15476286.2016.1244588. Epub 2016 Oct 11. RNA Biol. 2017. PMID: 27726526 Free PMC article. Review. - Mechanisms and regulation underlying membraneless organelle plasticity control.
Ismail H, Liu X, Yang F, Li J, Zahid A, Dou Z, Liu X, Yao X. Ismail H, et al. J Mol Cell Biol. 2021 Aug 4;13(4):239-258. doi: 10.1093/jmcb/mjab028. J Mol Cell Biol. 2021. PMID: 33914074 Free PMC article. Review. - A stress-activated, p38 mitogen-activated protein kinase-ATF/CREB pathway regulates posttranscriptional, sequence-dependent decay of target RNAs.
Gao J, Wagnon JL, Protacio RM, Glazko GV, Beggs M, Raj V, Davidson MK, Wahls WP. Gao J, et al. Mol Cell Biol. 2013 Aug;33(15):3026-35. doi: 10.1128/MCB.00349-13. Epub 2013 Jun 3. Mol Cell Biol. 2013. PMID: 23732911 Free PMC article. - To translate, or not to translate: viral and host mRNA regulation by interferon-stimulated genes.
Li MM, MacDonald MR, Rice CM. Li MM, et al. Trends Cell Biol. 2015 Jun;25(6):320-9. doi: 10.1016/j.tcb.2015.02.001. Epub 2015 Mar 3. Trends Cell Biol. 2015. PMID: 25748385 Free PMC article. Review.
References
- Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. - PubMed
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430–436. - PubMed
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10:1324–1332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources