Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates - PubMed (original) (raw)
Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates
Kvido Strisovsky et al. Mol Cell. 2009.
Abstract
Members of the widespread rhomboid family of intramembrane proteases cleave transmembrane domain (TMD) proteins to regulate processes as diverse as EGF receptor signaling, mitochondrial dynamics, and invasion by apicomplexan parasites. However, lack of information about their substrates means that the biological role of most rhomboids remains obscure. Knowledge of how rhomboids recognize their substrates would illuminate their mechanism and might also allow substrate prediction. Previous work has suggested that rhomboid substrates are specified by helical instability in their TMD. Here we demonstrate that rhomboids instead primarily recognize a specific sequence surrounding the cleavage site. This recognition motif is necessary for substrate cleavage, it determines the cleavage site, and it is more strictly required than TM helix-destabilizing residues. Our work demonstrates that intramembrane proteases can be sequence specific and that genome-wide substrate prediction based on their recognition motifs is feasible.
2009 Elsevier Inc.
Figures
Figure 1
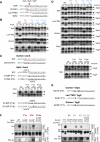
Diverse Bacterial Rhomboids Share Cleavage Site Specificity (A) Fusion proteins containing TMDs of TatA, Gurken, Spitz, and LacYTM2 were cleaved in vitro by bacterial rhomboids AarA (P. stuartii), GlpG (E. coli), and YqgP (B. subtilis). The Coomassie-stained gel shows the C-terminal proteolytic fragments (numbered) that were N-terminally sequenced by automated Edman degradation. The alignment of substrate sequences shows that all three rhomboids cleave each substrate in the same position (indicated by an arrow). TMDs as predicted by the program Phobius (Kall et al., 2004) are underlined, and TM helix-destabilizing residues are shown in bold. (B) Autoradiography shows that the in vitro-translated full-length TatA is cleaved by detergent-solubilized purified AarA in a time- and enzyme-concentration-dependent manner (in this and all subsequent figures, a black arrowhead indicates the substrate and an open arrowhead the cleaved product). Substrate conversion was quantified based on densitometric scanning of the autoradiogram. Data show mean values from four experiments ± standard deviation. (C) Deletions within the TatA TMD show that the intact hydrophobic domain is required for efficient cleavage. AarA concentration was 280 nM, and cleavage rates were evaluated as in (B).
Figure 2
Transmembrane Helix-Destabilizing Residues Are Required for Cleavage of TatA (A) TM helix-destabilizing residues G11, S12, P13, and Q15 in TatA were mutated into leucine in all combinations. The in vitro translated and radiolabeled mutant substrates were assayed for cleavage efficiency using 280 nM AarA and 40 min reaction time to ensure appropriate sensitivity (see Figure 1B). At least two TM helix destabilizers are required for efficient cleavage. WT, wild-type enzyme; SA, catalytic serine-to-alanine mutant. (B) The requirement is specific for residues with low TM helical propensity: substitution of the four TM helix destabilizers by other TM helix-stabilizing residues (alanine) completely blocks cleavage in vitro, whereas other TM helix-destabilizing residues (asparagine) allow substantial cleavage. The in vitro-translated radiolabeled proteins were cleaved by 280 nM AarA. Substrate conversion values were derived from the SDS PAGE gel autoradiogram. (C) The same trend as in (B) is observed in biological membranes. TatA and its mutants were overexpressed in wild-type P. stuartii expressing endogenous AarA. Each protein was purified from the membrane fraction through its C-terminal His tag and analyzed by MALDI mass spectrometry. The spectra show the relative proportion of full-length and AarA cleaved forms in each case. The N termini of individual species were inferred from their molecular mass, as indicated. The experimental masses of the uncleaved full-length proteins were consistent with the presence of N-terminal formylmethionine.
Figure 3
Factors Determining Cleavage Site Position (A) Preferences at P1 and P1′ positions in TatA. A8 and A9 were mutated individually into amino acids whose side chains represent a range of physicochemical properties and analyzed for cleavage by AarA. The P1 position is much more constrained than P1′: tolerated mutations are highlighted in boldface. Enzyme concentration was 450 nM and reaction time 40 min. WT, wild-type; SA, catalytic serine-to-alanine mutant. (B) A linker containing susceptible P1-P1′ pairs was inserted between G11 and S12 of TatA to generate the i7 mutant. As indicated by the large arrows, it is cleaved by AarA in vivo only at the original A8-A9 site. Cleavage also occurs at the same site in vitro, and when overdigested, less-efficient secondary cleavages occur in the linker region (small arrows). The theoretical masses of the C-terminal fragments resulting from i7 cleavage at indicated sites are annotated above its sequence. Upper graph, MALDI mass spectrum of the in vivo-processed i7 mutant that had been expressed in wild-type P. stuartii and isolated from the membrane fraction. Experimental masses of [M+H] ions and corresponding N termini are indicated. The N-terminal sequence determined by Edman degradation is highlighted in bold. Masses of the minor peaks marked with asterisks could not be matched to any cleavage product, and their identity was not established. Lower graph, MALDI mass spectra of an in vitro cleavage reaction time course of the i7 mutant that had been expressed in E. coli ΔglpG and isolated from the membrane fraction. AarA concentration was 11.2 μM, and recombinant i7 was at 20 μM. (C) Cleavage rate of linker insertion mutants in vitro is negatively proportional to linker length. AarA was at 280 nM. (D) TM helix-destabilizing residues are less important when cleavage occurs outside the bilayer. Substitution of S12, P13, and Q14 by leucine completely blocks cleavage in vitro in the context of otherwise wild-type TatA, but not in the context of the i7 mutant. To ensure comparable cleavage rates between the pairs, AarA concentration was 280 nM for WT and 3L, and 840 nM for i7 and i7/3L. (E) Cleavage rate comparison of TMD deletion variants of the i7 linker insertion mutant showed that even when cleavage occurs outside the TMD, the hydrophobic part of i7 TMD is still required. AarA concentration was 840 nM. In (C)–(E), within each experiment the in vitro-translated radiolabeled substrate variants were equimolar as judged by equal intensity of their bands on the autoradiograms, and the cleavage rates were quantitated as in Figure 1B.
Figure 4
TatA Cleavage Site Is Determined by a Three Amino Acid Recognition Motif (A) Importance of each residue within the TatA cleavage site region, encompassing P7–P7′, was examined by phenylalanine and glycine scanning mutagenesis. Enzyme concentration was 280 nM and reaction time 40 min (see Figure 1B). (B) The P5–P2′ region was further scrutinized by comprehensive positional scanning mutagenesis. The effects of the mutations were graded into four levels based on the mutant substrate conversion at the end of a 40 min reaction in comparison to the wild-type TatA. Enzyme concentration was 280 nM. Notably, the P1, P4, and P2′ positions in TatA are the most sensitive to mutations. A vertical arrow marks the site of cleavage by AarA. (C) Mutations in P4, P1, and P2′ of the TatA recognition motif that inhibit cleavage in vitro have equally strong inhibitory effect in biological membranes in vivo. TatA mutants were overexpressed in P. stuartii, isolated from membrane fraction, and N-terminally sequenced. MALDI mass spectra show relative proportions of full-length versus cleaved forms in each case with experimental molecular weights. The N termini corresponding to individual peak masses are indicated; those determined by Edman degradation are shown in bold. Minor peaks marked with asterisks could not correspond to any TatA cleavage product, since their mass was larger than that of full-length TatA; their identity was not established.
Figure 5
The TatA-like Recognition Motif in Substrates Is Required by Rhomboids from Evolutionarily Distant Species (A) The recognition motif occurs in all four substrates examined. Protein fragments are aligned by rhomboid cleavage site, and the crucial motif residues are highlighted: P1 in red, P4 and P2′ in blue. (B) Recognition motifs in TatA, LacYTM2, Gurken, and Spitz are required by AarA, and they can be disabled by point mutations. The substrates were in vitro translated and radiolabeled; the enzyme was used at 280, 140, 1120, and 224 nM, respectively; and reaction time was 40 min. Note that due to second translation initiation at an internal methionine residue, Spitz and Gurken may contain a weak band of similar mobility as the rhomboid cleavage product. WT, wild-type; SA, catalytic serine-to-alanine mutant. (C) Analysis of cleavage sites in Gurken P2′ and Spitz mutants by N-terminal sequencing and mass spectrometry. The I247G mutation in the P2′ of Gurken and A138F mutation in P1 of Spitz have created new recognition motifs (color coded; the introduced mutations in bold and italicized). Spitz contains a secondary recognition motif (b) that is used when the primary one (a) is knocked out by L135G mutation. Mutations of the P1a and P1b positions of Spitz into proline do not block cleavage individually, but they do so in combination. (D) Bacterial rhomboids GlpG and YqgP require identical recognition motifs in the same set of substrates. Purified GlpG was used at 0.8, 3.2, 6.4, and 0.8 μM, and detergent-solubilized E. coli membranes containing YqgP were used at 0.8, 0.2, 0.8, and 1.6 μg/μL for TatA, LacYTM2, Gurken, and Spitz, respectively. (E) Cleavage site analysis by N-terminal sequencing. GlpG is able to cleave a suboptimal version of the recognition motif in Gurken I247G with glycine in P2′. YqgP recognizes a secondary but completely stereotypic recognition motif in LacYTM2 P1 serine-to-phenylalanine mutant, but it can also cleave LacYTM2 and Gurken to some extent even with a phenylalanine in P1 position. (F) Western blots of cell-based cleavage assays showing that Drosophila Rhomboid-1 recognizes the same motifs in Spitz and Gurken that are required by bacterial rhomboids. Recognition motif mutations strongly inhibit cleavage of Gurken in cells and secretion of Gurken and Spitz into the media. Superfluous lanes have been cropped out from each gel for clarity (indicated by white lines).
Figure 6
Genome-wide Substrate Predictions Based on the Recognition Motif (A) The regular expression representing the AarA recognition motif derived from the specificity matrix (Figure 4B) that was used to search the P. stuartii subproteome of type I and III single-TMD proteins ([ ] matches any single alphabetical character contained within the brackets, and [ˆ ] matches any single alphabetical character not contained in the brackets). Analysis ranges for motif search were limited to “P” for predicted substrates and to “N” for predicted nonsubstrates. (B) Overall analysis workflow. (C) The top-scoring candidate substrates and predicted nonsubstrates were tested for cleavage by AarA in vitro. The protein-encoding fragments were amplified from P. stuartii genomic DNA and in vitro translated; AarA concentration in cleavage reactions was 560 nM, and reaction time 40 min. Open arrows denote cleavage products. WT, wild-type; SA, catalytic serine-to-alanine mutant; NCBI IDs for each protein are indicated.
Figure 7
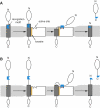
An Updated Model for Rhomboid Substrate Recognition We suggest that rhomboid substrates are defined by two main specificity-conferring elements. A substrate's TMD binds the intramembrane-located “exosite” on the rhomboid enzyme while the recognition motif has affinity to the solvent-exposed rhomboid active site region. Both elements are distinct and separable in a substrate's primary structure. (A) Substrates with a recognition motif located within or near the N terminus of their TMD require the presence of downstream TM helix-destabilizing residues that facilitate local unfolding; this allows the recognition motif access to the rhomboid active site. (B) Substrates with a recognition motif located outside the TMD do not require TM helix-destabilizing residues, since the linker region between the motif and the TMD is sufficient to allow the motif access to the rhomboid active site.
Comment in
- Rhomboid proteases: familiar features in unfamiliar phases.
Amarneh B, Rawson RB. Amarneh B, et al. Mol Cell. 2009 Dec 25;36(6):922-3. doi: 10.1016/j.molcel.2009.12.005. Mol Cell. 2009. PMID: 20064458
Similar articles
- Substrate specificity of rhomboid intramembrane proteases is governed by helix-breaking residues in the substrate transmembrane domain.
Urban S, Freeman M. Urban S, et al. Mol Cell. 2003 Jun;11(6):1425-34. doi: 10.1016/s1097-2765(03)00181-3. Mol Cell. 2003. PMID: 12820957 - Conservation of intramembrane proteolytic activity and substrate specificity in prokaryotic and eukaryotic rhomboids.
Urban S, Schlieper D, Freeman M. Urban S, et al. Curr Biol. 2002 Sep 3;12(17):1507-12. doi: 10.1016/s0960-9822(02)01092-8. Curr Biol. 2002. PMID: 12225666 - Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity.
Urban S, Wolfe MS. Urban S, et al. Proc Natl Acad Sci U S A. 2005 Feb 8;102(6):1883-8. doi: 10.1073/pnas.0408306102. Epub 2005 Jan 31. Proc Natl Acad Sci U S A. 2005. PMID: 15684070 Free PMC article. - Emerging role of rhomboid family proteins in mammalian biology and disease.
Bergbold N, Lemberg MK. Bergbold N, et al. Biochim Biophys Acta. 2013 Dec;1828(12):2840-8. doi: 10.1016/j.bbamem.2013.03.025. Epub 2013 Apr 3. Biochim Biophys Acta. 2013. PMID: 23562403 Review. - Structural and mechanistic principles of intramembrane proteolysis--lessons from rhomboids.
Strisovsky K. Strisovsky K. FEBS J. 2013 Apr;280(7):1579-603. doi: 10.1111/febs.12199. Epub 2013 Mar 20. FEBS J. 2013. PMID: 23432912 Review.
Cited by
- Differential processing of VesB by two rhomboid proteases in Vibrio cholerae.
Roberts CS, Shannon AB, Korotkov KV, Sandkvist M. Roberts CS, et al. mBio. 2024 Sep 11;15(9):e0127024. doi: 10.1128/mbio.01270-24. Epub 2024 Aug 13. mBio. 2024. PMID: 39136457 Free PMC article. - GlyGly-CTERM and rhombosortase: a C-terminal protein processing signal in a many-to-one pairing with a rhomboid family intramembrane serine protease.
Haft DH, Varghese N. Haft DH, et al. PLoS One. 2011;6(12):e28886. doi: 10.1371/journal.pone.0028886. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194940 Free PMC article. - Substrate-Enzyme Interactions in Intramembrane Proteolysis: γ-Secretase as the Prototype.
Liu X, Zhao J, Zhang Y, Ubarretxena-Belandia I, Forth S, Lieberman RL, Wang C. Liu X, et al. Front Mol Neurosci. 2020 May 19;13:65. doi: 10.3389/fnmol.2020.00065. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32508589 Free PMC article. Review. - Regulation of Peptidase Activity beyond the Active Site in Human Health and Disease.
Obaha A, Novinec M. Obaha A, et al. Int J Mol Sci. 2023 Dec 4;24(23):17120. doi: 10.3390/ijms242317120. Int J Mol Sci. 2023. PMID: 38069440 Free PMC article. Review. - Proteolytic Activation of Plant Membrane-Bound Transcription Factors.
De Backer J, Van Breusegem F, De Clercq I. De Backer J, et al. Front Plant Sci. 2022 Jun 14;13:927746. doi: 10.3389/fpls.2022.927746. eCollection 2022. Front Plant Sci. 2022. PMID: 35774815 Free PMC article. Review.
References
- Akiyama Y., Maegawa S. Sequence features of substrates required for cleavage by GlpG, an Escherichia coli rhomboid protease. Mol. Microbiol. 2007;64:1028–1037. - PubMed
- Chao J.R., Parganas E., Boyd K., Hong C.Y., Opferman J.T., Ihle J.N. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452:98–102. - PubMed
- Engelman D.M., Steitz T.A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Biophys. Chem. 1986;15:321–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases