The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy - PubMed (original) (raw)
The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy
Wei-Lien Yen et al. J Cell Biol. 2010.
Abstract
Macroautophagy is a catabolic pathway used for the turnover of long-lived proteins and organelles in eukaryotic cells. The morphological hallmark of this process is the formation of double-membrane autophagosomes that sequester cytoplasm. Autophagosome formation is the most complex part of macroautophagy, and it is a dynamic event that likely involves vesicle fusion to expand the initial sequestering membrane, the phagophore; however, essentially nothing is known about this process including the molecular components involved in vesicle tethering and fusion. In this study, we provide evidence that the subunits of the conserved oligomeric Golgi (COG) complex are required for double-membrane cytoplasm to vacuole targeting vesicle and autophagosome formation. COG subunits localized to the phagophore assembly site and interacted with Atg (autophagy related) proteins. In addition, mutations in the COG genes resulted in the mislocalization of Atg8 and Atg9, which are critical components involved in autophagosome formation.
Figures
Figure 1.
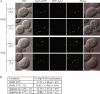
The Cvt, autophagy, and pexophagy pathways are defective in cog mutants. (A) The Cvt pathway is defective in COG deletion mutants. The wild-type (WT; BY4742), cog1Δ, cog5Δ, cog6Δ, cog7Δ, and cog8Δ cells were grown in rich medium, and rapamycin was added to the culture at a final concentration of 0.2 µg/ml. After 2 h, cell extracts were prepared and subjected to immunoblot analysis with anti-Ape1 or anti-YFP antisera. (B) Autophagic activity in the COG deletion mutants. The wild-type (YTS223), cog1Δ (YTS224), cog5Δ (YTS225), cog6Δ (YTS226), cog7Δ (YTS227), cog8Δ (YTS228), and atg1Δ (YTS243) cells expressing Pho8Δ60 were grown in YPD medium and incubated in SD-N for 3 h to induce autophagy. Cell extracts were prepared for measuring the Pho8Δ60-dependent alkaline phosphatase activity. Error bars indicate SD. (C) The Cvt pathway is defective in cog2-1 and cog3-2 mutants. The wild-type (BY4742), cog2-1, and cog3-2 cells were grown in rich medium at 24°C and shifted to SMD. After incubation at either 24 or 38°C for 30 min, cells were labeled with [35S]methionine/cysteine and subjected to a nonradioactive chase at the same temperature for 2 h. Ape1 was immunoprecipitated and subjected to SDS-PAGE. (D) GFP-Atg8 processing is defective in cog temperature-sensitive mutants. Wild-type (BY4742), cog2-1, cog3-2, cog4-1 (BCR29), and cog4-2 (BCR47) mutants were grown at permissive temperature until OD600 = 0.8. After incubation at either 24°C or nonpermissive temperatures (37 or 39°C) for 30 min, rapamycin was added for 2 h. Aliquots were collected, and protein extracts were subjected to immunoblotting analysis. (E) Autophagic activity in temperature-sensitive cog mutants. Wild-type (YTS223), cog2-1 (YTS230), cog3-2 (YTS231), and cog2-1 and cog3-2 strains harboring the pCuCOG2HA(416) and pCuCOG3HA(416) plasmids, respectively, expressing Pho8Δ60 were grown in YPD medium and incubated in SD-N at either 24 or 37°C for 3 h. Cell lysates were used to measure Pho8Δ60 activity. (F) COG complex subunits are required for pexophagy. The wild-type (IRA001), atg1Δ (IRA002), cog2-1 (WLY90), and cog3-2 (WLY91) cells expressing Pex14-GFP were grown under conditions to induce peroxisome proliferation as described in Materials and methods. After incubation at either 24°C or nonpermissive temperatures for 30 min, the cultures were shifted to SD-N. For cog1Δ (WLY175) and cog6Δ (WLY174) strains, the cells were grown at 30°C and after being induced for peroxisome proliferation were shifted to SD-N at the same temperature. Protein extracts were prepared at the indicated time points and resolved by SDS-PAGE.
Figure 2.
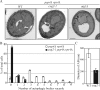
Anterograde movement of Atg9 is defective in the cog2-1 strain. cog2-1 Atg9-3GFP (WLY227) cells expressing RFP-Ape1 were grown to OD600 = 0.8 at 24°C or shifted to 37°C for 1 h. For autophagy-inducing conditions, rapamycin was added for 1 h at either 24 or 37°C before imaging. DIC, differential interference contrast. Bar, 2.5 µm. (B) Quantification of Atg9 PAS localization. The colocalization percentages were quantified in cells containing both Atg9-3GFP and RFP-Ape1 signals from three independent repeat experiments.
Figure 3.
The cog mutants are defective in Atg8 localization to the PAS/phagophore. GFP-Atg8 localization is defective in cog2-1 and cog3-2 mutants. The wild-type (WT; BY4742) and cog2-1 strains carrying a plasmid expressing GFP-Atg8 (pCuGFP-AUT7(416)) were grown in SMD at 24°C to OD600 = 0.8 or shifted to nonpermissive temperature for 1 h before imaging. For starvation conditions, the cells were incubated at either 24°C or nonpermissive temperature for 60 min, rapamycin (final concentration 0.2 µg/ml) was added, and the culture was incubated for another 30 min before imaging. To reverse the temperature, cultures were shifted back to 24°C for 30 min in both growing and rapamycin-treated conditions. Essentially, the same results as shown for cog2-1 were seen with the cog3-2 mutant. DIC, differential interference contrast. Bars, 2.5 µm.
Figure 4.
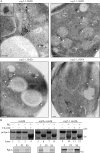
The cog mutants affect sequestering vesicle formation. (A) cog2-1 cells were grown in SMD and shifted to 38°C in SMD or SD-N for 1.5 h. The cells were analyzed by immunoelectron microscopy and labeled with anti-YFP antibody followed by immunogold as described in Materials and methods. The white arrowheads mark clusters of GFP-Atg8, and the black arrow marks a phagophore. Bars, 200 nm. The inset shows an image immunostained with affinity-purified antiserum to Ape1, demonstrating that the electron-dense structure is a Cvt complex. Bar, 100 nm. (B) Cog2 is involved in Cvt vesicle formation. Spheroplasts from the wild-type (vam3Δ; WLY118), cog2-1 (WLY117), and atg1Δ (WLY119) strains were incubated at 37°C for 20 min, pulse labeled with [35S]methionine/cysteine for 10 min, and subjected to a nonradioactive chase for 30 min. The prApe1-containing pellet fractions were obtained after osmotically lysing the spheroplasts and centrifugation at 5,000 g. The pellets were treated with proteinase K (PK) in the presence or absence of 0.2% Triton X-100 for 20 min on ice. The resulting samples were TCA precipitated and immunoprecipitated with Ape1 or Pgk1 antiserum and resolved by SDS-PAGE. T, total; P5 and S5, pellet and supernatant fractions, respectively, after 5,000 g centrifugation.
Figure 5.
The COG complex is required for autophagosome formation. (A) The wild-type (WT; pep4Δ vps4Δ; FRY143), atg1Δ (JHY28), and cog2-1 (WLY221) strains were grown in YPD at 24°C to OD600 = 0.8, shifted to SD-N for 1.5 h at 37°C, and prepared for electron microscopy analysis as described in Materials and methods. Bars, 0.5 µm. (B) Quantification of autophagic body accumulation. The number of autophagic bodies in 54 or 75 cells containing vacuoles of similar size in wild-type and cog2-1 cells, respectively, was quantified. (C) Quantification of the diameter of autophagic bodies (AB). The diameter of autophagic bodies in wild-type (n = 45) and cog2-1 (n = 37) cells was measured and quantified. Error bars represent SD.
Figure 6.
Cog2 localizes to the PAS. (A) Cog2-GFP (WLY190) and cog2-1–GFP (WLY188) strains expressing RFP-Ape1 were grown in SMD at 24°C or shifted to 37°C for 30 min before imaging. For autophagy-inducing conditions, cells were either grown at 24°C or shifted to 37°C for 30 min, and rapamycin was added for an additional 30 min. DIC, differential interference contrast. Bars, 2.5 µm. (B) Quantification of Cog2-GFP and cog2-1–GFP PAS localization. The colocalization percentages were quantified in cells containing both GFP and RFP-Ape1 signals from three independent repeat experiments.
Figure 7.
COG subunits associate with Atg proteins. (A and B) HA-Cog4 (A; WLY208) and HA-Cog2 (B; WLY209) cells transformed with the indicated plasmids expressing tagged Atg proteins were grown in selective SMG medium to OD600 = 1.0. Cell lysates were prepared and subjected to affinity isolation or immunoprecipitation (IP) with either anti-HA or anti-Myc antibody as described in Materials and methods. Plasmids expressing PA (pRS424-CuProtA), PA-tagged Atg17 (pProtA-Apg17(424)), Atg20 (pProtA-Cvt20(424)), Atg24 (pProtA-Cvt13(424)), and Myc-tagged Atg12 (pMyc-Apg12(426)) were used as indicated. The eluted proteins were separated by SDS-PAGE and detected with monoclonal anti-HA antibody (A) or immunoblotting with anti-HA and anti-Myc antibodies (B). For each experiment, ∼1% of the total cell lysate or 10% of the total eluate was loaded. WB, Western blot. (C) Cog2-GFP colocalizes with RFP-Ape1 in the MKO (ATG11 ATG19) strain. The MKO (ATG11 ATG19; WLY205) cells expressing chromosomally tagged Cog2-GFP and a plasmid-based RFP-Ape1 were grown in selective SMD to OD600 = 0.8 and observed by fluorescence microscopy. DIC, differential interference contrast. Bar, 2.5 µm.
Similar articles
- A nuclear membrane-derived structure associated with Atg8 is involved in the sequestration of selective cargo, the Cvt complex, during autophagosome formation in yeast.
Baba M, Tomonaga S, Suzuki M, Gen M, Takeda E, Matsuura A, Kamada Y, Baba N. Baba M, et al. Autophagy. 2019 Mar;15(3):423-437. doi: 10.1080/15548627.2018.1525475. Epub 2018 Oct 11. Autophagy. 2019. PMID: 30238844 Free PMC article. - Atg9 vesicles recruit vesicle-tethering proteins Trs85 and Ypt1 to the autophagosome formation site.
Kakuta S, Yamamoto H, Negishi L, Kondo-Kakuta C, Hayashi N, Ohsumi Y. Kakuta S, et al. J Biol Chem. 2012 Dec 28;287(53):44261-9. doi: 10.1074/jbc.M112.411454. Epub 2012 Nov 5. J Biol Chem. 2012. PMID: 23129774 Free PMC article. - Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae.
Geng J, Nair U, Yasumura-Yorimitsu K, Klionsky DJ. Geng J, et al. Mol Biol Cell. 2010 Jul 1;21(13):2257-69. doi: 10.1091/mbc.e09-11-0969. Epub 2010 May 5. Mol Biol Cell. 2010. PMID: 20444978 Free PMC article. - Mechanistic Insights into the Role of Atg11 in Selective Autophagy.
Zientara-Rytter K, Subramani S. Zientara-Rytter K, et al. J Mol Biol. 2020 Jan 3;432(1):104-122. doi: 10.1016/j.jmb.2019.06.017. Epub 2019 Jun 22. J Mol Biol. 2020. PMID: 31238043 Free PMC article. Review. - Phagophore closure, autophagosome maturation and autophagosome fusion during macroautophagy in the yeast Saccharomyces cerevisiae.
Kraft C, Reggiori F. Kraft C, et al. FEBS Lett. 2024 Jan;598(1):73-83. doi: 10.1002/1873-3468.14720. Epub 2023 Aug 23. FEBS Lett. 2024. PMID: 37585559 Review.
Cited by
- Hypothesis: determining phenotypic specificity facilitates understanding of pathophysiology in rare genetic disorders.
Haijes HA, Jaeken J, van Hasselt PM. Haijes HA, et al. J Inherit Metab Dis. 2020 Jul;43(4):701-711. doi: 10.1002/jimd.12201. Epub 2020 Jan 17. J Inherit Metab Dis. 2020. PMID: 31804708 Free PMC article. - Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis.
Dupont N, Chauhan S, Arko-Mensah J, Castillo EF, Masedunskas A, Weigert R, Robenek H, Proikas-Cezanne T, Deretic V. Dupont N, et al. Curr Biol. 2014 Mar 17;24(6):609-20. doi: 10.1016/j.cub.2014.02.008. Epub 2014 Mar 6. Curr Biol. 2014. PMID: 24613307 Free PMC article. - Atg9 proteins, not so different after all.
Ungermann C, Reggiori F. Ungermann C, et al. Autophagy. 2018;14(8):1456-1459. doi: 10.1080/15548627.2018.1477382. Epub 2018 Jul 23. Autophagy. 2018. PMID: 29966469 Free PMC article. - Atg23 and Atg27 act at the early stages of Atg9 trafficking in S. cerevisiae.
Backues SK, Orban DP, Bernard A, Singh K, Cao Y, Klionsky DJ. Backues SK, et al. Traffic. 2015 Feb;16(2):172-90. doi: 10.1111/tra.12240. Epub 2014 Dec 16. Traffic. 2015. PMID: 25385507 Free PMC article. - A High Copy Suppressor Screen for Autophagy Defects in _Saccharomyces arl1_Δ and _ypt6_Δ Strains.
Yang S, Rosenwald A. Yang S, et al. G3 (Bethesda). 2017 Feb 9;7(2):333-341. doi: 10.1534/g3.116.035998. G3 (Bethesda). 2017. PMID: 27974437 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases