Neuronal polarity - PubMed (original) (raw)
Review
Neuronal polarity
Sabina Tahirovic et al. Cold Spring Harb Perspect Biol. 2009 Sep.
Abstract
The assembly of functional neuronal networks in the developing animal relies on the polarization of neurons, i.e., the formation of a single axon and multiple dendrites. Breaking the symmetry of neurons depends on cytoskeletal rearrangements. In particular, axon specification requires local dynamic instability of actin and stabilization of microtubules. The polarized cytoskeleton also provides the basis for selective trafficking and retention of cellular components in the future somatodendritic or axonal compartments. Hence, these mechanisms are not only essential to achieve neuronal polarization, but also to maintain it. Different extracellular and intracellular signals converge on the regulation of the cytoskeleton. Most notably, Rho GTPases, PI3K, Ena/VASP, cofilin and SAD kinases are major intracellular regulators of neuronal polarity. Analyzing polarity signals under physiological conditions will provide a better understanding of how neurons can be induced to repolarize under pathological conditions, i.e., to regenerate their axons after central nervous system (CNS) injury.
Figures
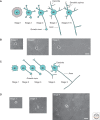
Figure 1.
Neuronal polarization in cultured neurons. Developmental stages in cultured rodent embryonic hippocampal neurons (A, B) and postnatal cerebellar granule neurons (C, D). (A) Hippocampal neurons transform from round cells bearing lamellipodia (Stage 1) into multipolar cells (Stage 2). One neurite enlarges its growth cone and extends rapidly to become the axon (Stage 3). The remaining shorter neurites will develop into dendrites (Stage 4). This is followed by functional maturation and formation of dendritic spines and synapses (Stages 5–6). (B) Phase-contrast images of cultured hippocampal neurons in stages 1, 2, and 3. Scale bar, 20µm. (C) Shortly after plating, cerebellar granule neurons protrude several filopodia (Stage 1) and then start extending one process (Stage 2). From the opposite side of the cell body, another process develops: The granule neuron adopts bipolar morphology bearing two axons (Stage 3). One of the two axons elongates further and starts branching (Stage 4), while the other axon retracts and shorter dendrites form around the cell body (Stage 5). (D) Phase-contrast images of cultured cerebellar granule neurons in stages 1, 3, and 4. Scale bar, 20 µm.
Figure 2.
The growth cone cytoskeletal structure. (A) Illustration of an axonal growth cone. Microtubules (red) distributed along the axonal shaft protrude into the central region of the growth cone. The growth cone is enriched in F-actin (green) that is organized into long bundles forming filamentous protrusions, filopodia, or veil-like sheets of branched actin forming lamellipodia. Lamellipodia and filopodia are important for growth cone dynamics. (B) Immunocytochemical image of a stage-2 hippocampal neuron bearing an enlarged growth cone in one of the neurites. The actin cytoskeleton is stained in green and microtubules in red.
Figure 3.
Intracellular mechanisms driving neuronal polarization. Morphologically unpolarized neurons bear several equal neuronal processes. (A) In one of these processes, intracellular signaling pathways, leading to axon formation, are activated and result in early changes in cytoskeleton dynamics. (B) This neurite starts elongating rapidly and a morphologically polarized neuron bearing an axon is formed. (C) The intracellular symmetry-breaking events are represented schematically: (1) centrosome (yellow) as the potential spatial signal for axon initiation; (2) cytoskeletal changes: the actin cytoskeleton (green) in the axonal growth cone is more dynamic and microtubules (red) are more stable in comparison to minor neurites; (3) stable microtubules are recognized by particular kinesin motors (orange) that induce unidirectional membrane trafficking (green) toward the axon. This leads to molecular segregation of cellular components and neuronal polarization. Reprinted, with permission from Witte and Bradke 2008.
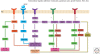
Figure 4.
Coordination of extracellular and intracellular signaling regulates cytoskeletal dynamics and axon formation. Overview of selected signaling pathways that may initiate neuronal polarization and axon specification. BDNF signaling (blue) leads to activation of PKA/LKB1/SAD A/B signaling that regulates the stability of microtubules and induces axon formation. The PI3K/PIP3/Akt/GSK-3β pathway (purple) regulates MAPs and the stability of microtubules. Another signaling branch activates Rap1B and the Rho GTPases Cdc42 and Rac1. Signals from both of these GTPases regulate actin dynamics via WASP/WAVE (red) or PAK-cofilin pathways (green). Rac1 may also regulate microtubules by regulating the microtubule destabilizer Op18/Stathmin. Apart from being downstream of PI3K signaling, Cdc42 and Rac1 may be activated by other signals (potential green and red plasma membrane [PM] receptors). Another molecule implicated in axon development is RhoA, which might regulate actin via the LIMK/cofilin pathway (green) or microtubule dynamics via ROCK signaling to MAPs (orange).
Similar articles
- The role of the cytoskeleton during neuronal polarization.
Witte H, Bradke F. Witte H, et al. Curr Opin Neurobiol. 2008 Oct;18(5):479-87. doi: 10.1016/j.conb.2008.09.019. Epub 2008 Oct 25. Curr Opin Neurobiol. 2008. PMID: 18929658 Review. - Neuronal polarization: the cytoskeleton leads the way.
Stiess M, Bradke F. Stiess M, et al. Dev Neurobiol. 2011 Jun;71(6):430-44. doi: 10.1002/dneu.20849. Dev Neurobiol. 2011. PMID: 21557499 Review. - Crucial polarity regulators in axon specification.
Lalli G. Lalli G. Essays Biochem. 2012;53:55-68. doi: 10.1042/bse0530055. Essays Biochem. 2012. PMID: 22928508 Review. - Molecular mechanisms of axon specification and neuronal disorders.
Yoshimura T, Arimura N, Kaibuchi K. Yoshimura T, et al. Ann N Y Acad Sci. 2006 Nov;1086:116-25. doi: 10.1196/annals.1377.013. Ann N Y Acad Sci. 2006. PMID: 17185510 Review. - Roles of Rho-family GTPases in cell polarisation and directional migration.
Fukata M, Nakagawa M, Kaibuchi K. Fukata M, et al. Curr Opin Cell Biol. 2003 Oct;15(5):590-7. doi: 10.1016/s0955-0674(03)00097-8. Curr Opin Cell Biol. 2003. PMID: 14519394 Review.
Cited by
- Anesthetics interfere with the polarization of developing cortical neurons.
Mintz CD, Smith SC, Barrett KM, Benson DL. Mintz CD, et al. J Neurosurg Anesthesiol. 2012 Oct;24(4):368-75. doi: 10.1097/ANA.0b013e31826a03a6. J Neurosurg Anesthesiol. 2012. PMID: 23085784 Free PMC article. - Patterning skin by planar cell polarity: the multi-talented hair designer.
Chen J, Chuong CM. Chen J, et al. Exp Dermatol. 2012 Feb;21(2):81-5. doi: 10.1111/j.1600-0625.2011.01425.x. Exp Dermatol. 2012. PMID: 22229440 Free PMC article. - Calcineurin-dependent cofilin activation and increased retrograde actin flow drive 5-HT-dependent neurite outgrowth in Aplysia bag cell neurons.
Zhang XF, Hyland C, Van Goor D, Forscher P. Zhang XF, et al. Mol Biol Cell. 2012 Dec;23(24):4833-48. doi: 10.1091/mbc.E12-10-0715. Epub 2012 Oct 24. Mol Biol Cell. 2012. PMID: 23097492 Free PMC article. - Structural Basis of the Avian Influenza NS1 Protein Interactions with the Cell Polarity Regulator Scribble.
Javorsky A, Humbert PO, Kvansakul M. Javorsky A, et al. Viruses. 2022 Mar 11;14(3):583. doi: 10.3390/v14030583. Viruses. 2022. PMID: 35336989 Free PMC article. - Golgi during development.
Zhong W. Zhong W. Cold Spring Harb Perspect Biol. 2011 Sep 1;3(9):a005363. doi: 10.1101/cshperspect.a005363. Cold Spring Harb Perspect Biol. 2011. PMID: 21768608 Free PMC article. Review.
References
- Andersen SS, Bi GQ 2000. Axon formation: A molecular model for the generation of neuronal polarity. Bioessays 22:172–9 - PubMed
- Arimura N, Kaibuchi K 2005. Key regulators in neuronal polarity. Neuron 48:881–884 - PubMed
- Arimura N, Kaibuchi K 2007. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat Rev Neurosci 8:194–205 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources