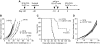CD137 agonist antibody prevents cancer recurrence: contribution of CD137 on both hematopoietic and nonhematopoietic cells - PubMed (original) (raw)
CD137 agonist antibody prevents cancer recurrence: contribution of CD137 on both hematopoietic and nonhematopoietic cells
Hidehiko Narazaki et al. Blood. 2010.
Abstract
Antigen-specific memory T cells (Tms) are essential in the immune surveillance of residual and metastatic tumors. Activation of Tms requires designing vaccines based on tumor rejection antigens, which are often not available to cancer patients. Therefore, it is desirable to have a general applicable approach to activate Tms without extensive knowledge of tumor antigens. Here, we report that activation of antigen-specific Tms could be achieved by the administration of agonistic anti-CD137 monoclonal antibody without additional tumor vaccination, leading to the prevention of recurrence and metastases after surgical resection of primary tumors in mouse models. By reconstitution with CD137-deficient Tms, we demonstrate that expression of CD137 on antigen-specific Tms is only partially required for the effect of anti-CD137 antibody. Other host cells, including those from hematopoietic and nonhematopoietic origins, are also important because ablation of CD137 from these cells partially but significantly eliminates antitumor effect of anti-CD137 antibody. Our findings implicate a potential new approach to prevent recurrence and metastases in cancer patients.
Figures
Figure 1
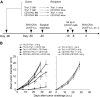
Effect of anti-CD137 mAb in the prevention of recurrence and metastases of murine B16-OVA melanoma. (A) A schematic of mouse models of melanoma recurrence and metastases and anti-CD137 mAb treatment protocol. (B) Effect of anti-CD137 mAb in the prevention of recurrence of B16-OVA tumor. Upon removal of primary tumors, mice were challenged with B16-OVA tumor cells subcutaneously, and the sizes of tumors were measured individually and recorded as the mean tumor diameter regularly. Results are 1 representative of 3 independent experiments. (C) Effect of anti-CD137 mAb in the prevention of metastases of B16-OVA tumor. Upon removal of primary tumors, mice were challenged with B16-OVA tumor cells intravenously, and the survival of mice was monitored daily up to 120 days. Results are 1 representative of 3 independent experiments. ***Significantly different from control mAb (rat Ig) treatment group, P < .001. (D) Presensitization of mice to primary tumors is required for the effect of anti-CD137 mAb. Mice were mock-treated by surgery, as in panel B but without primary tumor inoculation, and subsequently treated with anti-CD137 mAb. B16-OVA tumors were then inoculated, measured individually, and recorded as the mean tumor diameter regularly. Results are 1 representative of 3 independent experiments.
Figure 2
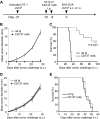
Effect of anti-CD137 mAb in the stimulation of Tms to prevent tumor outgrowth in the OT-1 adoptive transfer model. (A) A schematic of the treatment of a melanoma mouse model with Tms and anti-CD137 mAb. In vitro–activated OT-1 TCR-transgenic T cells were transferred into mice for the development of Tms. (B) Effect of anti-CD137 mAb in preventing B16-OVA tumor challenge. One month after transfer of activated OT-1 T cells, mice were treated with anti-CD137 mAb and subsequently challenged with B16-OVA tumor cells subcutaneously. The sizes of tumors were measured individually and recorded as the mean tumor diameter regularly. Results are 1 representative of 4 independent experiments. Each point is the mean ± SD of tumor diameters in a group of 5 mice. (C) Effect of anti-CD137 mAb in the prevention of metastases of B16-OVA tumor. One month after transfer of activated OT-1 T cells, mice were treated with anti-CD137 mAb and subsequently challenged with B16-OVA tumor cells intravenously. The survival of mice was monitored daily up to 100 days. Results are 1 representative of 3 independent experiments. ***Significantly different from control mAb (rat Ig) treatment group, P < .001. (D-E) Effect of anti-CD137 mAb fails to prevent tumor challenge after transfer of naive OT-1 cells. The procedure is the same as in panel A, but naive instead of activated OT-1 T cells were transferred. Tumor growth (D) and survival (E) of the mice were monitored as described in panels B-C. Results are 1 representative of 2 independent experiments.
Figure 3
Flow cytometric analysis of mononuclear cells after anti-CD137 mAb treatment. Naive B6 mice (Thy1.2) were transferred with 107 purified activated (A) or naive (B) OT-1 T cells (CD8+ Thy1.1). As previously described, 3 weeks later they were treated with rat Ig or anti-CD137 mAb. At 10 days after antibody treatment, mononuclear cells were harvested from spleen, lymph nodes (axilla and inguinal), and peripheral blood. OT-1 T cells were identified by anti-Thy1.1 mAb. Absolute numbers of OT-1 T cells of spleen, lymph nodes, and peripheral blood were calculated. Subsets of effector Tms (CD44hiCD62Llo), central Tms (CD44hiCD62Lhi), and naive T cells (CD44lo) of OT-1 T cells were identified by the indicated specific mAbs. Results are 1 representative of at least 3 independent experiments. ns indicates not significant; *P < .05; ***P < .005.
Figure 4
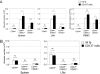
Treatment by anti-CD137 mAb promotes functions of memory OT-1 T cells in vivo. (A) In vivo CTL assay. Naive B6 spleen cells were labeled with CFSE at a high (5μM, CFSEhi, right peaks) or low dose (0.5μM, CFSElo, left peaks) and pulsed with OVA (SIINFEKL) or the control peptide, respectively, as target cells. Target cells at 1 × 107 were injected intravenously into the recipient mice 1 month after transfer of activated OT-1 cells and treatment with anti-CD137 mAb or control Ig. Splenocytes were collected from the recipient mice for detection of CFSE-labeled cells by flow cytometry at 6 and 24 hours. Percentage lysis of peptide-pulsed target cells was calculated from the ratio of CFSEhi/CFSElo target cells. (B) ELISPOT assays for IFN-γ–producing cells. ELISPOT assays were performed in triplicate, using spleen cells with OVA peptide or mitomycin C–treated B16-OVA tumor cells. The spots that represent IFN-γ–producing cells were counted, and the mean values ± SD are shown. Results represent 3 independent experiments. *P < .05; **P < .01.
Figure 5
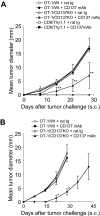
CD137 expression on both OT-1 Tms and host cells is required for the effect of anti-CD137 mAb. (A) CD137 on OT-1 Tms is required for the effect of anti-CD137 mAb. Spleen cells were prepared from wild-type OT-1 (OT-1/Wt) or CD137-deficient OT-1 (OT-1/CD137KO) mice, and activated by anti-CD3/CD28 mAb in vitro for 2 days. In addition, spleen cells from Thy1.1 mice (CD8+Thy1.1) were also activated with the same procedure as controls. Activated cells were transferred into naive B6 mice for memory T-cell development. One month later, mice were treated with rat Ig or anti-CD137 mAb as indicated. One week after antibody treatment, B16-OVA was inoculated subcutaneously. Tumor sizes were measured regularly. Each point is the mean (± SD) tumor diameter in a group of 5 mice, and the result is a representative of 3 independent experiments. **P < .01 compared with the OT-1/Wt Tms treated with control rat Ig. Mice treated with OT-1/CD137KO Tms and anti-CD137 mAb had a significantly weaker response against tumor in comparison with those treated with OT-1/Wt Tms and anti-CD137 mAb (P = .026, 2-way ANOVA). (B) CD137 on host cells is required for the effect of anti-CD137 mAb. OT-1/Wt and OT-1/CD137KO were transferred into naive CD137KO mice and subsequently treated with rat Ig or anti-CD137 mAb. Each point indicates the mean tumor diameters in a group of 5 mice, and error bars show SD. Results are 1 representative of 3 independent experiments. *P < .05 compared with the OT-1/CD137KO treated with anti-CD137 mAb.
Figure 6
Both hematopoietic and nonhematopoietic host cells are required for the antirecurrence/metastases effect of anti-CD137 mAb. (A) A schematic of the mouse model for melanoma recurrence/metastases after bone marrow (BM) reconstitution and anti-CD137 mAb treatment. At day −90, indicated recipient mice were irradiated with 650 Gy twice on the same day. BM cells were harvested from the indicated donor mice and transferred at 5 × 106/mouse into indicated recipient mice. The percentage of bone marrow chimerism was evaluated 30 days after BM transplantation. Chimeric mice were inoculated with 2 × 105 B16-OVA tumor cells subcutaneously at day −50. Ten days later, tumor nodules were surgically resected. Mice rested for 1 month to facilitate the development of memory T cells and were subsequently treated with anti-CD137 mAb twice as indicated. At day 0, mice were rechallenged with B16-OVA tumor cells at 2 × 105 subcutaneously or intravenously. (B) BM-reconstituted and tumor-resected mice were treated with control rat Ig or anti-CD137 mAb (left panel) or control rat Ig (right panel), and B16-OVA tumor cells were inoculated subcutaneously 1 week later. Each point indicates the mean tumor diameters in a group of 5 mice, and error bars show SD. *P < .05, **P < .01 compared with Thy1.2BM/Thy1.1 host treated with control rat Ig. +P < .05, ++P < .01 compared with Thy1.2BM/Thy1.1 host treated with anti-CD137 mAb.
Similar articles
- Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion.
Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, Torchia JA, Kolstad A, Chen L, Levy R. Houot R, et al. Blood. 2009 Oct 15;114(16):3431-8. doi: 10.1182/blood-2009-05-223958. Epub 2009 Jul 29. Blood. 2009. PMID: 19641184 Free PMC article. - Epitope and Fc-Mediated Cross-linking, but Not High Affinity, Are Critical for Antitumor Activity of CD137 Agonist Antibody with Reduced Liver Toxicity.
Ho SK, Xu Z, Thakur A, Fox M, Tan SS, DiGiammarino E, Zhou L, Sho M, Cairns B, Zhao V, Xiong M, Samayoa J, Forsyth CM, Powers DB, Chao DT, Hollenbaugh D, Alvarez HM, Akamatsu Y. Ho SK, et al. Mol Cancer Ther. 2020 Apr;19(4):1040-1051. doi: 10.1158/1535-7163.MCT-19-0608. Epub 2020 Jan 23. Mol Cancer Ther. 2020. PMID: 31974274 - [The immunotherapy potential of agonistic anti-CD137 (4-1BB) monoclonal antibodies for malignancies and chronic viral diseases].
Alfaro C, Murillo O, Tirapu I, Azpilicueta A, Huarte E, Arina A, Arribillaga L, Pérez-Gracia JL, Bendandi M, Prieto J, Lasarte JJ, Melero I. Alfaro C, et al. An Sist Sanit Navar. 2006 Jan-Apr;29(1):77-96. doi: 10.4321/s1137-66272006000100007. An Sist Sanit Navar. 2006. PMID: 16670731 Review. Spanish. - Antitumor efficacy of CD137 ligation is maximized by the use of a CD137 single-chain Fv-expressing whole-cell tumor vaccine compared with CD137-specific monoclonal antibody infusion.
Zhang H, Knutson KL, Hellstrom KE, Disis ML, Hellstrom I. Zhang H, et al. Mol Cancer Ther. 2006 Jan;5(1):149-55. doi: 10.1158/1535-7163.MCT-05-0206. Mol Cancer Ther. 2006. PMID: 16432173 - Therapeutic vaccination with tumor cells that engage CD137.
Hellstrom KE, Hellstrom I. Hellstrom KE, et al. J Mol Med (Berl). 2003 Feb;81(2):71-86. doi: 10.1007/s00109-002-0413-8. Epub 2003 Feb 8. J Mol Med (Berl). 2003. PMID: 12601523 Review.
Cited by
- S100A4 blockage alleviates agonistic anti-CD137 antibody-induced liver pathology without disruption of antitumor immunity.
Zhang J, Song K, Wang J, Li Y, Liu S, Dai C, Chen L, Wang S, Qin Z. Zhang J, et al. Oncoimmunology. 2018 Jan 23;7(4):e1296996. doi: 10.1080/2162402X.2017.1296996. eCollection 2018. Oncoimmunology. 2018. PMID: 29632708 Free PMC article. - CD137 expression is induced by Epstein-Barr virus infection through LMP1 in T or NK cells and mediates survival promoting signals.
Yoshimori M, Imadome K, Komatsu H, Wang L, Saitoh Y, Yamaoka S, Fukuda T, Kurata M, Koyama T, Shimizu N, Fujiwara S, Miura O, Arai A. Yoshimori M, et al. PLoS One. 2014 Nov 19;9(11):e112564. doi: 10.1371/journal.pone.0112564. eCollection 2014. PLoS One. 2014. PMID: 25409517 Free PMC article. - Delivering safer immunotherapies for cancer.
Milling L, Zhang Y, Irvine DJ. Milling L, et al. Adv Drug Deliv Rev. 2017 May 15;114:79-101. doi: 10.1016/j.addr.2017.05.011. Epub 2017 May 22. Adv Drug Deliv Rev. 2017. PMID: 28545888 Free PMC article. Review. - CD137-mediated pathogenesis from chronic hepatitis to hepatocellular carcinoma in hepatitis B virus-transgenic mice.
Wang J, Zhao W, Cheng L, Guo M, Li D, Li X, Tan Y, Ma S, Li S, Yang Y, Chen L, Wang S. Wang J, et al. J Immunol. 2010 Dec 15;185(12):7654-62. doi: 10.4049/jimmunol.1000927. Epub 2010 Nov 8. J Immunol. 2010. PMID: 21059892 Free PMC article. - Circulating CD137+ T Cell Levels Are Correlated with Response to Pembrolizumab Treatment in Advanced Head and Neck Cancer Patients.
Cirillo A, Zizzari IG, Botticelli A, Strigari L, Rahimi H, Scagnoli S, Scirocchi F, Pernazza A, Pace A, Cerbelli B, d'Amati G, Marchetti P, Nuti M, Rughetti A, Napoletano C. Cirillo A, et al. Int J Mol Sci. 2023 Apr 12;24(8):7114. doi: 10.3390/ijms24087114. Int J Mol Sci. 2023. PMID: 37108276 Free PMC article.
References
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76(6):959–962. - PubMed
- Armitage RJ. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol. 1994;6(3):407–413. - PubMed
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–620. - PubMed
- Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H. CD137 expression in tumor vessel walls: high correlation with malignant tumors. Am J Clin Pathol. 2001;115(4):543–549. - PubMed
- Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–4888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA106861-02/CA/NCI NIH HHS/United States
- CA-106861/CA/NCI NIH HHS/United States
- R01 AI072592-04/AI/NIAID NIH HHS/United States
- R01 CA106861-04/CA/NCI NIH HHS/United States
- R01 AI072592/AI/NIAID NIH HHS/United States
- R01 AI072592-02/AI/NIAID NIH HHS/United States
- R01 CA106861-03/CA/NCI NIH HHS/United States
- R01 CA106861/CA/NCI NIH HHS/United States
- AI-072592/AI/NIAID NIH HHS/United States
- R01 AI072592-01/AI/NIAID NIH HHS/United States
- R01 CA106861-01/CA/NCI NIH HHS/United States
- R01 AI072592-05/AI/NIAID NIH HHS/United States
- R01 CA106861-06/CA/NCI NIH HHS/United States
- R01 CA106861-05/CA/NCI NIH HHS/United States
- R01 AI072592-03/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials