Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke - PubMed (original) (raw)
Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke
Haihao Zhu et al. J Cereb Blood Flow Metab. 2010 Jun.
Abstract
Recent studies showed that soluble annexin A2 dramatically increases tissue plasminogen activator (tPA)-mediated plasmin generation in vitro, and reduces thrombus formation in vivo. Here, we hypothesize that combining annexin A2 with tPA can significantly enhance thrombolysis efficacy, so that lower doses of tPA can be applied in ischemic stroke to avoid neurotoxic and hemorrhagic complications. In vitro activity assays confirmed tPA-specific amplification of plasmin generation by recombinant annexin A2. In a rat focal embolic stroke model, combination therapy with tPA and recombinant annexin A2 protein at 2 h post-ischemia decreased the effective dose required for tPA by four-fold and reduced brain infarction. Combining annexin A2 with tPA also lengthened the time window for thrombolysis. Compared with tPA (10 mg/kg) alone, the combination of annexin A2 (5 mg/kg) plus low-dose tPA (2.5 mg/kg) significantly enhanced fibrinolysis, attenuated mortality, brain infarction, and hemorrhagic transformation, even when administered at 4 h post-ischemia. Combination with recombinant annexin A2, the effective thrombolytic dose of tPA can be decreased. As a result, brain hemorrhage and infarction are reduced, and the time window for stroke reperfusion prolonged. Our present findings provide a promising new approach for enhancing tPA-based thrombolytic stroke therapy.
Figures
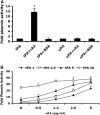
Figure 1
Effect of rA2 on tPA-dependent plasmin generation in vitro. (A) Plasmin activity was measured as described under Materials and methods and expressed as a ratio to that generated by either 2.5 _μ_g/mL of tPA alone or 100 Units/mL of uPA alone. Concentrations of both rA2 and BSA were 5 _μ_g/mL. Data were expressed as mean+s.e.m., *P<0.001, _n_=4 per group. (B) A range of concentrations of tPA (1, 2.5, 5, 10 _μ_g/mL) with or without the indicated concentrations of rA2 (0, 1, 2.5, 5 _μ_g/mL) were added to wells of 96-well plate. Plasmin activity was represented as fold of plasmin activity related to 1 _μ_g/mL of tPA alone. Data were expressed as mean+s.e.m., _n_=4 per group.
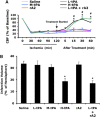
Figure 2
Effect of treating rats at 2 h after initiation of ischemia. (A) Two hours after initiation of ischemia, animals were treated intravenously with saline, high-dose tPA (10 mg/kg, H-tPA), intermediate-dose tPA (5 mg/kg, M-tPA), low-dose tPA (2.5 mg/kg, L-tPA), rA2 alone (5 mg/kg), or a combination of low-dose tPA (2.5 mg/kg) plus rA2 (5 mg/kg). Laser-doppler flowmetry was used to monitor regional cerebral blood flow (rCBF) for up to 1 h after treatment. (B) At 24 h after stroke, brain infarction was stained by TTC, and the volume was quantified using computer-assisted image analysis. Data were expressed as mean+s.e.m., *P<0.05 for L-tPA plus rAN, #P<0.05 for H-tPA, respectively, _n_=7 or 8 per group.
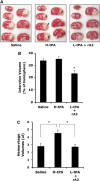
Figure 3
Effect of treating rats at 4 h after initiation of ischemia. (A) Representative images of brain sections after TTC staining at 24 h after initiating ischemia. At 4 h after stroke onset, three groups of rats were treated intravenously with saline, high-dose tPA (10 mg/kg, H-tPA), or low-dose tPA (2.5 mg/kg, L-tPA) plus rA2 (5 mg/kg). Ischemic infarctions (white color area) were detected in all three groups; however, large areas of grossly visible hemorrhage appeared only on the brain sections of H-tPA-treated rats pointed by arrows. (B) At 24 h after stroke, brain infarction was quantified using computer-assisted image analysis. (C) Volumes of intracerebral hemorrhage ware quantified with hemoglobin assay at 24 h after stroke. Data were expressed as mean+s.e.m., *P<0.05, _n_=13 for saline, _n_=10 for H-tPA, _n_=14 for the combination.
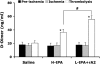
Figure 4
Effect of tPA alone or in combination with rA2 on plasma levels of -dimer. Plasma samples were collected before ischemia, just before thrombolytic therapy, and 1 h after treatment. Concentrations of -dimer in plasma were quantified by ELISA analysis. Data were expressed as mean+s.e.m., *P<0.01 versus ischemia, #P<0.01, _n_=6 per group.
Figure 5
MMP activation in ischemic brains from rats treated 4 h after stroke onset. At 24 h after stroke onset, we performed in situ zymography to examine MMP activation in ischemic brains at delayed 4 h treatments. In the cortex of the periinfarction zone, brains from animals treated with tPA alone showed brighter activated MMP signals compared with saline treatment, but the combination of low-dose tPA plus rA2 had similar or even slightly less positive signals compared with tPA alone treatment. Similar observations were obtained from three individual experiments.
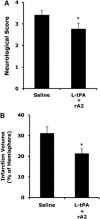
Figure 6
Three days neurological outcomes in rats treated at 4 h after stroke onset. (A) Two groups of rats were treated intravenously at 4 h after stroke with either saline, or a combination of low-dose tPA (2.5 mg/kg, L-tPA) plus rA2 (5 mg/kg). Three days after stroke, neurological scores were significantly improved in treated rats. (B) Ischemic infarctions on H&E-stained sections were quantified using computer-assisted image analysis. Infarction volumes were significantly reduced in the treated rats. *P<0.05, _n_=8 for saline, _n_=11 for the combination.
Similar articles
- Low dose tPA plus annexin A2 combination attenuates tPA delayed treatment-associated hemorrhage and improves recovery in rat embolic focal stroke.
Jiang Y, Fan X, Yu Z, Cheng C, Wang XS, Lo EH, Sun X, Wang X. Jiang Y, et al. Neurosci Lett. 2015 Aug 18;602:73-8. doi: 10.1016/j.neulet.2015.06.050. Epub 2015 Jul 3. Neurosci Lett. 2015. PMID: 26149229 Free PMC article. - Annexin A2 Plus Low-Dose Tissue Plasminogen Activator Combination Attenuates Cerebrovascular Dysfunction After Focal Embolic Stroke of Rats.
Fan X, Jiang Y, Yu Z, Liu Q, Guo S, Sun X, van Leyen K, Ning M, Gao X, Lo EH, Wang X. Fan X, et al. Transl Stroke Res. 2017 Dec;8(6):549-559. doi: 10.1007/s12975-017-0542-6. Epub 2017 Jun 4. Transl Stroke Res. 2017. PMID: 28580536 - Effects of tissue plasminogen activator and annexin A2 combination therapy on long-term neurological outcomes of rat focal embolic stroke.
Wang X, Fan X, Yu Z, Liao Z, Zhao J, Mandeville E, Guo S, Lo EH, Wang X. Wang X, et al. Stroke. 2014 Feb;45(2):619-22. doi: 10.1161/STROKEAHA.113.003823. Epub 2013 Dec 24. Stroke. 2014. PMID: 24368559 Free PMC article. - Combination Low-Dose Tissue-Type Plasminogen Activator Plus Annexin A2 for Improving Thrombolytic Stroke Therapy.
Jiang Y, Fan X, Yu Z, Liao Z, Wang XS, van Leyen K, Sun X, Lo EH, Wang X. Jiang Y, et al. Front Cell Neurosci. 2015 Oct 14;9:397. doi: 10.3389/fncel.2015.00397. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26528130 Free PMC article. Review. - The annexin A2 system and vascular homeostasis.
Flood EC, Hajjar KA. Flood EC, et al. Vascul Pharmacol. 2011 Mar-Jun;54(3-6):59-67. doi: 10.1016/j.vph.2011.03.003. Epub 2011 Mar 29. Vascul Pharmacol. 2011. PMID: 21440088 Free PMC article. Review.
Cited by
- Intravenous tPA therapy does not worsen acute intracerebral hemorrhage in mice.
Foerch C, Rosidi NL, Schlunk F, Lauer A, Cianchetti FA, Mandeville E, Arai K, Yigitkanli K, Fan X, Wang X, van Leyen K, Steinmetz H, Schaffer CB, Lo EH. Foerch C, et al. PLoS One. 2013;8(2):e54203. doi: 10.1371/journal.pone.0054203. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23408937 Free PMC article. - Recombinant Tissue Plasminogen Activator-conjugated Nanoparticles Effectively Targets Thrombolysis in a Rat Model of Middle Cerebral Artery Occlusion.
Deng J, Mei H, Shi W, Pang ZQ, Zhang B, Guo T, Wang HF, Jiang XG, Hu Y. Deng J, et al. Curr Med Sci. 2018 Jun;38(3):427-435. doi: 10.1007/s11596-018-1896-z. Epub 2018 Jun 22. Curr Med Sci. 2018. PMID: 30074208 - Improved thrombolytic effect with focused ultrasound and neuroprotective agent against acute carotid artery thrombosis in rat.
Lee TH, Yeh JC, Tsai CH, Yang JT, Lou SL, Seak CJ, Wang CY, Wei KC, Liu HL. Lee TH, et al. Sci Rep. 2017 May 9;7(1):1638. doi: 10.1038/s41598-017-01769-2. Sci Rep. 2017. PMID: 28487554 Free PMC article. - Feedback regulation of endothelial cell surface plasmin generation by PKC-dependent phosphorylation of annexin A2.
He KL, Sui G, Xiong H, Broekman MJ, Huang B, Marcus AJ, Hajjar KA. He KL, et al. J Biol Chem. 2011 Apr 29;286(17):15428-39. doi: 10.1074/jbc.M110.185058. Epub 2010 Nov 29. J Biol Chem. 2011. PMID: 21115493 Free PMC article. - Danhong Injection Combined With t-PA Improves Thrombolytic Therapy in Focal Embolic Stroke.
Li M, Zhou J, Jin W, Li X, Zhang Y. Li M, et al. Front Pharmacol. 2018 Apr 6;9:308. doi: 10.3389/fphar.2018.00308. eCollection 2018. Front Pharmacol. 2018. PMID: 29681849 Free PMC article.
References
- Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–e534. - PubMed
- Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. - PubMed
- Armstead WM, Cines DB, Higazi AA. Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig. Stroke. 2005;36:2265–2269. - PubMed
- Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab. 2009;29:1463–1474. - PMC - PubMed
- Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, Higazi AA. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci. 2006;9:1150–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS065998/NS/NINDS NIH HHS/United States
- P01-046403/PHS HHS/United States
- R01 NS048422/NS/NINDS NIH HHS/United States
- P50-NS10828/NS/NINDS NIH HHS/United States
- R01-NS48422/NS/NINDS NIH HHS/United States
- R01-NS37074/NS/NINDS NIH HHS/United States
- P50 NS010828/NS/NINDS NIH HHS/United States
- R01 HL042493/HL/NHLBI NIH HHS/United States
- R01-HL042493/HL/NHLBI NIH HHS/United States
- R01 NS049476/NS/NINDS NIH HHS/United States
- R01-NS53560/NS/NINDS NIH HHS/United States
- R01 HL090895/HL/NHLBI NIH HHS/United States
- R01-NS049476/NS/NINDS NIH HHS/United States
- R01 NS037074/NS/NINDS NIH HHS/United States
- R01 NS053560/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials