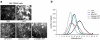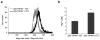TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase - PubMed (original) (raw)
TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase
Li-Ting Su et al. J Mol Biol. 2010.
Abstract
TRPM7 is a Ca(2)(+)-permeant and Mg(2)(+)-permeant ion channel in possession of its own kinase domain. In a previous study, we showed that overexpression of the channel-kinase in HEK-293 cells produced cell rounding and loss of adhesion, which was dependent on the Ca(2+)-dependent protease m-calpain. The TRPM7-elicited change in cell morphology was channel-dependent and occurred without any significant increase in cytosolic Ca(2+). Here we demonstrate that overexpression of TRPM7 increased levels of cellular reactive oxygen species (ROS) and nitric oxide, causing the activation of p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK). Application of inhibitors of p38 MAPK and JNK blocked TRPM7-induced cell rounding and activation of m-calpain, without affecting the phosphorylation state of the protease. Overexpression of TRPM7 increased intracellular Mg(2+); however, when the concentration of either external Ca(2+) or Mg(2+) was increased to favor the permeation of one divalent cation over the other, a similar increase in cell rounding and calpain activity was detected, indicating that TRPM7-mediated activation of m-calpain is not dependent on the nature of the divalent conducted by the channel. Application of inhibitors of nitric oxide synthase and mitochondrial-derived ROS reduced TRPM7-induced increases in nitric oxide and ROS production, blocked the change in cell morphology, and reduced cellular calpain activity. Collectively, our data reveal that excessive TRPM7 channel activity causes oxidative and nitrosative stresses, producing cell rounding mediated by p38 MAPK/JNK-dependent activation of m-calpain.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures
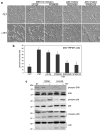
Figure 1
Overexpression of TRPM7 in HEK-293 cells activates p38 MAPK and JNK, but suppresses ERK. (a) Application of the p38 MAPK inhibitor SB203580 (1 μM) and JNK inhibitor SP600125 (20 μM) to 293-TRPM7 cells treated with tetracycline (+TET) for 24 hours reduced TRPM7-induced cell rounding to levels similar to cells grown in the absence of tetracycline. In contrast, treatment of cells with the MEK1/2 inhibitors U0126 (5 μM) and PD98059 (10 μM) had only a partial effect. Scale bar is equal to 100 μm. (b) Quantification of the degree of cell rounding under the conditions depicted in (a). Values are mean ± standard deviation of at least three independent experiments. A χ2 test was employed to assess differences in cell rounding between 293-TRPM7 cells treated with the different inhibitors. An asterisk indicates treatments that produced a decrease in cell rounding that was significantly different from 293-TRPM7 cells grown in tetracycline. (c) Western blots showing expression of TRPM7 or kinase-inactive TRPM7-G1618D mutant (+TET) caused a modest increase in p38 MAPK and JNK activities and a reduction in ERK activation. “NT” is non-transfected HEK-293 cells.
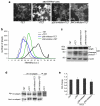
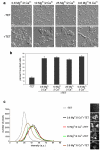
Figure 2
p38 MAPK and JNK are required to stimulate m-calpain activity when TRPM7 is overexpressed. (a) Calpain activity assay using fluorogenic BOC-LM-CMAC substrates. Overexpression of TRPM7 (+TET) stimulated intracellular cleavage of the synthetic calpain substrate producing greater cellular fluorescence emission compared to control cells (−TET). Treatment of 293-TRPM7 expressing cells with p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 inhibited the TRPM7-dependent increase in fluorescence emission. Scale bar is equal to 100 μm. The experiment was repeated three times with similar results. (b) The intensity of fluorescence emission from cells under the conditions depicted in (a) was quantified and displayed as a histogram of the distribution of intensity. The x-axis represents fluorescence emission intensity and the y-axis indicates the number of pixels found for each respective intensity. (c) Western blots using a monoclonal antibody that recognizes the head domain of talin was used to probe cell lysates from 293-TRPM7 cells treated with tetracycline in the presence of various inhibitors. Expression of TRPM7 caused an increase in the cleavage of m-calpain substrate talin into its head and rod domains. The head domain cleavage product migrated at 47 kDa. Application of p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 reduced proteolysis of talin in response to TRPM7 expression. A western blot of β-actin is shown to demonstrate equal loading of samples. (d) In vivo [32P]orthophosphate labeling of 293-TRPM7 cells treated with tetracycline in the presence of various inhibitors. Shown is an autoradiograph of incorporation of [32P] into m-calpain immunoprecipitated from cell lysates. An anti-m-calpain blot is shown to demonstrate the quantity of immunoprecipitated protein. (e) Quantification of [32P]orthophosphate incorporation normalized to m-calpain protein. Results are presented as fold change compared to the negative control (−TET). Values are mean ± standard deviation of five independent experiments.
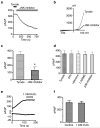
Figure 3
The effects of SAPK, mitochondrial, and NOS inhibitors on TRPM7 channel activity. (a) Application of the JNK inhibitor SP600126 (20 μM) decreased TRPM7 current amplitude over time. (b) Representative trace showing TRPM7 current-voltage relationship before and after application of the JNK inhibitor. (c) TRPM7 outward current was blocked by the JNK inhibitor by approximately 63% at +100 mV (N=5). The asterisk indicates a significant difference in TRPM7 current compared to the negative control (Tyrode) using a Student t test (p<0.01). (d) TRPM7 current density at +100 mV was not changed by the application of DPI (1 μM), rotenone (100 nM), L-NAME (2 mM), and the p38 inhibitor SB203580 (40 μM) (N=4). (e) Representative trace showing that application of 1 mM H2O2 did not change TRPM7 inward or outward conductance over time. (f) TRPM7 current density at +100 mV was unchanged by the application of 1 mM H2O2 (N=8).
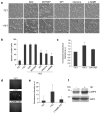
Figure 4
The effects of ROS scavengers or inhibitors of mitochondrial- and NOS-derived free radicals on TRPM7-mediated cell rounding. (a) Application of ROS scavenger NAC (5 μM) and MnTBAP (200 μM) had no effect on the TRPM7-induced cell rounding. In contrast, application of DPI (0.2 μM) and rotenone (10 nM) attenuated cell rounding. Treatment of cells with NOS inhibitor L-NAME (2 mM) also reduced cell rounding. Scale bar is equal to 100 μm. (b) Quantification of the degree of cell rounding under the conditions depicted in (a). An asterisk indicates treatments that produced a decrease in cell rounding that were significantly different from 293-TRPM7 cells grown in tetracycline using χ2 test. (c) Measurement of ROS generation in 293-TRPM7 cells. Overexpression of TRPM7 (+TET) resulted in a modest increase in ROS generation compared to control cells (−TET) that can be reversed with DPI. Average of fluorescence intensity was normalized to control cells (−TET). Values are mean ± standard deviation of three independent experiments. The asterisk indicates a signification difference in fluorescence intensity compare to control cells using a Student t test (p<0.05). (d) Fluorescence emission of 293-TRPM7 cells labeled with the nitric oxide (NO) indicator DAF-FM diacetate. Overexpression of TRPM7 (+TET) resulted in an increase in NO over non-expressing cells (−TET) that can be reversed with L-NAME (2 mM). (e) Quantification of the fluorescence intensity under the condition depicted in (d). Values are mean ± standard deviation of three independent experiments. The asterisk indicates a signification difference in fluorescence intensity compared to the negative control (−TET) using a Student t test (p<0.05). (f) Western blot with an antibody against AMP-dependent kinase (AMPK) and the Thr172-phosphorylated “activated-form” of AMPK indicates that overexpression of TRPM7 increases AMPK activation by 44%. The AMPK experiments were repeated three times with similar results.
Figure 5
Inhibitors of ROS production and NOS attenuate TRPM7-dependent elevation of cellular calpain activity. (a) Calpain activity assay showing application of DPI, rotenone, and L-NAME caused a decrease in fluorescence emission intensity in 293-TRPM7 expressing cells (+TET). Scale bar is equal to 100 μm. (b) The intensity of fluorescence emission from cells under the conditions depicted in (a) was quantified and displayed as a histogram of the distribution of intensity as described in Figure 2b. The calpain activity assay was repeated three times with similar results.
Figure 6
TRPM7-mediated cell rounding is independent of the divalent cation permeated by the channel. (a) Cell rounding analysis of 293-TRPM7 expressing cells (+TET) after 24 hours of culture in regular DMEM media, which contained 0.8 mM Mg2+/ 2 mM Ca2+, or regular DMEM media with a total of 10 mM Mg2+, 25 mM Mg2+ or 5 mM Ca2+. (b) Quantification of the degree of cell rounding under the conditions depicted in (a). (c) Calpain activity assay of 293-TRPM7 expressing cells (+TET) under the conditions described in (a). Scale bar is equal to 100 μm. The experiments were repeated three times with similar results.
Figure 7
Overexpression of TRPM7 elevates cellular Mg2+ levels. (a) Measurement of intracellular free Mg2+ in 293-TRPM7 cells. Cells were loaded with the fluorescent ratiometric indicator Mag-Indo-1. Baseline Mag-Indo violet to Mag-Indo blue ratios were measured using a Beckman Coulter MoFlo XDP cell sorter. Fluorescence emission ratios were displayed using a histogram. Overexpression of TRPM7 (+TET) caused an increase in intracellular free Mg2+ compared to control cells (293-TRPM7-TET). Data are representative of five independent experiments. (b) ICP-MS quantitation of total Mg2+ in HNO3 extracts of 293-TRPM7 cells with and without tetracycline treatment, where average concentration of total cellular Mg2+ was calculated by normalizing to a [K+] of 120 mM. Values are mean ± standard deviation of at least two independent experiments. The asterisk indicates a significant difference in total Mg2+ compared to the negative control (293-TRPM7-TET) using a Student t test (p<0.05).
Similar articles
- A key role for Mg(2+) in TRPM7's control of ROS levels during cell stress.
Chen HC, Su LT, González-Pagán O, Overton JD, Runnels LW. Chen HC, et al. Biochem J. 2012 Aug 1;445(3):441-8. doi: 10.1042/BJ20120248. Biochem J. 2012. PMID: 22587440 Free PMC article. - TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain.
Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. Su LT, et al. J Biol Chem. 2006 Apr 21;281(16):11260-70. doi: 10.1074/jbc.M512885200. Epub 2006 Jan 25. J Biol Chem. 2006. PMID: 16436382 Free PMC article. - TRPM2 contributes to LPS/IFNγ-induced production of nitric oxide via the p38/JNK pathway in microglia.
Miyake T, Shirakawa H, Kusano A, Sakimoto S, Konno M, Nakagawa T, Mori Y, Kaneko S. Miyake T, et al. Biochem Biophys Res Commun. 2014 Feb 7;444(2):212-7. doi: 10.1016/j.bbrc.2014.01.022. Epub 2014 Jan 22. Biochem Biophys Res Commun. 2014. PMID: 24462864 - Silencing TRPM7 in mouse cortical astrocytes impairs cell proliferation and migration via ERK and JNK signaling pathways.
Zeng Z, Leng T, Feng X, Sun H, Inoue K, Zhu L, Xiong ZG. Zeng Z, et al. PLoS One. 2015 Mar 23;10(3):e0119912. doi: 10.1371/journal.pone.0119912. eCollection 2015. PLoS One. 2015. PMID: 25799367 Free PMC article. - TRPM7 and TRPM2-Candidate susceptibility genes for Western Pacific ALS and PD?
Hermosura MC, Garruto RM. Hermosura MC, et al. Biochim Biophys Acta. 2007 Aug;1772(8):822-35. doi: 10.1016/j.bbadis.2007.02.008. Epub 2007 Feb 24. Biochim Biophys Acta. 2007. PMID: 17395433 Free PMC article. Review.
Cited by
- Magnesium and the Hallmarks of Aging.
Dominguez LJ, Veronese N, Barbagallo M. Dominguez LJ, et al. Nutrients. 2024 Feb 9;16(4):496. doi: 10.3390/nu16040496. Nutrients. 2024. PMID: 38398820 Free PMC article. Review. - Procaspase activating compound 1 controls tetracycline repressor-regulated gene expression system.
Song C, Kim N, Park M, Lee J, Oh KB, Sim T. Song C, et al. Biosci Rep. 2019 Jan 8;39(1):BSR20180793. doi: 10.1042/BSR20180793. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30538170 Free PMC article. - The anti-tumour compound, RH1, causes mitochondria-mediated apoptosis by activating c-Jun N-terminal kinase.
Park MT, Song MJ, Oh ET, Lee H, Choi BH, Jeong SY, Choi EK, Park HJ. Park MT, et al. Br J Pharmacol. 2011 Jun;163(3):567-85. doi: 10.1111/j.1476-5381.2011.01233.x. Br J Pharmacol. 2011. PMID: 21250978 Free PMC article. - Suppression of renal TRPM7 may alleviate kidney injury in the renal transplantation.
Meng Z, Cao R, Wang Y, Cao H, Liu T, Yang Z, Wang X. Meng Z, et al. World J Urol. 2014 Oct;32(5):1303-11. doi: 10.1007/s00345-013-1208-y. Epub 2013 Nov 21. World J Urol. 2014. PMID: 24258314 - Role of TRP channels in the cardiovascular system.
Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L. Yue Z, et al. Am J Physiol Heart Circ Physiol. 2015 Feb 1;308(3):H157-82. doi: 10.1152/ajpheart.00457.2014. Epub 2014 Nov 21. Am J Physiol Heart Circ Physiol. 2015. PMID: 25416190 Free PMC article. Review.
References
- Nadler MJS, Hermosura MC, Inabe K, Perraud A-L, Zhu Q, Stokes AJ, Kurosaki T, Kine J-P, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. - PubMed
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. - PubMed
- Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005;15:667–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous