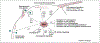An emerging role for p21-activated kinases (Paks) in viral infections - PubMed (original) (raw)
Review
An emerging role for p21-activated kinases (Paks) in viral infections
Celine Van den Broeke et al. Trends Cell Biol. 2010 Mar.
Abstract
p21-activated protein kinases (Paks) are cytosolic serine/threonine protein kinases that act as effectors for small (p21) GTPases of the Cdc42 and Rac families. It has long been established that Paks play a major role in a host of vital cellular functions such as proliferation, survival and motility, and abnormal Pak function is associated with a number of human diseases. Here, we discuss emerging evidence that these enzymes also play a major role in the entry, replication and spread of many important pathogenic human viruses, including HIV. Careful assessment of the potential role of Paks in antiviral immunity will be pivotal to evaluate thoroughly the potential of agents that inhibit Pak as a new class of anti-viral therapeutics.
Figures
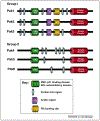
Figure 1.
Structural comparison of group I and group II Paks. Group I Pak members share a high degree of homology. The N-terminal part constitutes the regulatory region and contains the p21-binding domain that overlaps with the autoinhibitory domain. The C terminal part has a kinase domain that is more than 90% conserved among Pak I members. Group II Paks have the same domains, but they lack the autoinhibitory domain. The kinase domain of group II Paks is less conserved than that of group I Pak members.
Figure 2.
Pak1 and Pak2 amino acid sequence alignment. Conserved residues are marked by stars. The PxxP motif is responsible for binding of the adaptor protein Nck, whereas the CRIB motif is responsible for binding Cdc42. Delimitation between the regulatory and the catalytic region is shown by brackets. The arrow indicates the aspartic acid at position 212 that identifies the caspase cleavage site in Pak2, but not in Pak1. Binding of Nef to Pak is not dependent on the presence of the PxxP motif, Pix binding site, CRIB motif or the caspase cleavage site within Pak2. The Pak sequence important for Nef binding is underlined in red.
Figure 3.
Virus-mediated activation of Pak. The figure shows the different virus families, and if known the virus protein and downstream signaling cascades, that lead to Pak activation at some point during the virus replication cycle. The best-studied viral activation of Paks takes place in HIV infection where Paks are activated by the virus Nef protein via a Nef–Vav–Rac1–Cdc42 complex. Pak, p21-activated kinase; PI3K, phosphoinositide 3-kinase; Hbx, Hepatitis B virus X protein; GPCR, G-protein-coupled receptor; VV, vaccinia virus; EV1, echovirus 1; HIV, human immunodeficiency virus; PRV, pseudorabies virus; KSHV, Kaposi’s sarcoma-associated herpesvirus.
Figure 4.
Consequences of virus-mediated activation of Pak. Activation of Pak by a wide variety of viruses may lead to (i) cytoskeletal rearrangements such as cortical actin disassembly, macropinocytotic virus uptake, actin stress fiber disassembly and projection formation, (ii) anti-apoptotic signaling, (iii) oncogenesis, (iv) immune evasion and (v) antiviral signaling. Pak, p21-activated kinase; PRV, pseudorabies virus; HSV, herpes simplex virus; HIV, human immunodeficiency virus; EV1, echovirus 1; HCV, hepatitis C virus; KSHV, Kaposi’s sarcoma-associated herpesvirus; HBV, hepatitis B virus; VV, vaccinia virus; Ad3, adenovirus 3; DC, dendritic cell.
Similar articles
- PAK thread from amoeba to mammals.
Kumar A, Molli PR, Pakala SB, Bui Nguyen TM, Rayala SK, Kumar R. Kumar A, et al. J Cell Biochem. 2009 Jul 1;107(4):579-85. doi: 10.1002/jcb.22159. J Cell Biochem. 2009. PMID: 19350548 Free PMC article. Review. - CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1.
Lu X, Wu X, Plemenitas A, Yu H, Sawai ET, Abo A, Peterlin BM. Lu X, et al. Curr Biol. 1996 Dec 1;6(12):1677-84. doi: 10.1016/s0960-9822(02)70792-6. Curr Biol. 1996. PMID: 8994833 - Group I p21-activated kinases: emerging roles in immune function and viral pathogenesis.
Pacheco A, Chernoff J. Pacheco A, et al. Int J Biochem Cell Biol. 2010 Jan;42(1):13-6. doi: 10.1016/j.biocel.2009.09.006. Epub 2009 Sep 9. Int J Biochem Cell Biol. 2010. PMID: 19747561 Free PMC article. Review. - Biology of the p21-activated kinases.
Bokoch GM. Bokoch GM. Annu Rev Biochem. 2003;72:743-81. doi: 10.1146/annurev.biochem.72.121801.161742. Epub 2003 Mar 27. Annu Rev Biochem. 2003. PMID: 12676796 Review. - The Role of p21-Activated Kinases in Cancer and Beyond: Where Are We Heading?
Liu H, Liu K, Dong Z. Liu H, et al. Front Cell Dev Biol. 2021 Mar 16;9:641381. doi: 10.3389/fcell.2021.641381. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33796531 Free PMC article. Review.
Cited by
- Epstein-Barr virus transcytosis through polarized oral epithelial cells.
Tugizov SM, Herrera R, Palefsky JM. Tugizov SM, et al. J Virol. 2013 Jul;87(14):8179-94. doi: 10.1128/JVI.00443-13. Epub 2013 May 22. J Virol. 2013. PMID: 23698302 Free PMC article. - SARS-CoV-2/COVID-19: Viral Genomics, Epidemiology, Vaccines, and Therapeutic Interventions.
Uddin M, Mustafa F, Rizvi TA, Loney T, Suwaidi HA, Al-Marzouqi AHH, Eldin AK, Alsabeeha N, Adrian TE, Stefanini C, Nowotny N, Alsheikh-Ali A, Senok AC. Uddin M, et al. Viruses. 2020 May 10;12(5):526. doi: 10.3390/v12050526. Viruses. 2020. PMID: 32397688 Free PMC article. Review. - Association between p21 Ser31Arg polymorphism and the development of cervical lesion in women infected with high risk HPV.
Lima G, Santos E, Angelo H, Oliveira M, Heráclio S, Leite F, de Melo C, Crovella S, Maia M, Souza P. Lima G, et al. Tumour Biol. 2016 Aug;37(8):10935-41. doi: 10.1007/s13277-016-4979-0. Epub 2016 Feb 17. Tumour Biol. 2016. PMID: 26886286 - IL-9 aggravates SARS-CoV-2 infection and exacerbates associated airway inflammation.
Sadhu S, Dalal R, Dandotiya J, Binayke A, Singh V, Tripathy MR, Das V, Goswami S, Kumar S, Rizvi ZA, Awasthi A. Sadhu S, et al. Nat Commun. 2023 Jul 10;14(1):4060. doi: 10.1038/s41467-023-39815-5. Nat Commun. 2023. PMID: 37429848 Free PMC article. - Group I p21-activated kinases facilitate Tax-mediated transcriptional activation of the human T-cell leukemia virus type 1 long terminal repeats.
Chan CP, Siu YT, Kok KH, Ching YP, Tang HM, Jin DY. Chan CP, et al. Retrovirology. 2013 Apr 26;10:47. doi: 10.1186/1742-4690-10-47. Retrovirology. 2013. PMID: 23622267 Free PMC article.
References
- Radtke K et al. (2006) Viral interactions with the cytoskeleton: a hitchhiker’s guide to the cell. Cell Microbiol. 8, 387–400 - PubMed
- Manser E et al. (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 - PubMed
- Pandey A et al. (2002) Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene 21, 3939–3948 - PubMed
- Jaffer ZM and Chernoff J (2002) p21-activated kinases: three more join the Pak. Int J. Biochem. Cell Biol. 34, 713–717 - PubMed
- Arias-Romero LE and Chernoff J (2008) A tale of two Paks. Biol Cell 100, 97–108 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous