Evolutionary gain of function for the ER membrane protein Sec62 from yeast to humans - PubMed (original) (raw)
Evolutionary gain of function for the ER membrane protein Sec62 from yeast to humans
Linda Müller et al. Mol Biol Cell. 2010.
Abstract
Because of similarity to their yeast orthologues, the two membrane proteins of the human endoplasmic reticulum (ER) Sec62 and Sec63 are expected to play a role in protein biogenesis in the ER. We characterized interactions between these two proteins as well as the putative interaction of Sec62 with ribosomes. These data provide further evidence for evolutionary conservation of Sec62/Sec63 interaction. In addition, they indicate that in the course of evolution Sec62 of vertebrates has gained an additional function, the ability to interact with the ribosomal tunnel exit and, therefore, to support cotranslational mechanisms such as protein transport into the ER. This view is supported by the observation that Sec62 is associated with ribosomes in human cells. Thus, the human Sec62/Sec63 complex and the human ER membrane protein ERj1 are similar in providing binding sites for BiP in the ER-lumen and binding sites for ribosomes in the cytosol. We propose that these two systems provide similar chaperone functions with respect to different precursor proteins.
Figures
Figure 1.
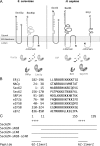
Structural and functional features of human Sec62 and Sec63. (A) Domain organization of Sec62, Sec63, and ERj1. Sec63 and ERj1 are membrane resident Hsp40s with ER lumenal J-domains and cochaperones for ER-lumenal Hsp70s (BiP and Kar2p). Sil1, Grp170, and Lhs1p act as nucleotide exchange factors for these Hsp70s. We note that loss of Sil1 function is linked to the human neurodegenerative disease Marinesco-Sjögren syndrome and may be nonlethal due to functional redundancy of Sil1 with Grp170 (Zimmermann et al., 2006). (B) Basic oligopeptides that are present in Sec62 and established ribosomal ligands in Homo sapiens. (C) Recombinant derivatives of human Sec62 and synthetic basic oligopeptides that are used throughout this study.
Figure 2.
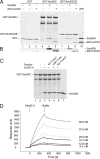
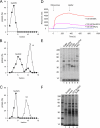
Human Sec62N interacts with human Sec63C. (A and B) GST or the two GST hybrid proteins, GST-Sec63C or GST-Sec63C26, were immobilized on GSH-Sepharose. Buffer, Sec62N, or Sec62N-ΔN10-ΔC40 (1 μM) was applied to the resin. After washing, the bound material was eluted and analyzed by SDS-PAGE as followed by either protein staining (A) or Western blotting plus immunodetection with anti-penta-histidine antibodies (B). Twenty-five percent of the input of the two Sec62 derivatives were run on the stained gel for comparison (A, lane 10). (C) GST-Sec63C hybrid was immobilized. Buffer, Sec62N (1 μM), or Sec62N in combination with the indicated oligopeptide (225 μM) was applied to the resin. After washing, the bound material was eluted and analyzed by SDS-PAGE followed by protein staining. Aliquots of the input of GST-Sec63 (lane 1) and Sec62 (lane 7) were run on the same gel for comparison. iv62-11mer, oligopeptide from invertebrate Sec62. (D) SPR analysis of the Sec62/Sec63 interaction. Human Sec62N was immobilized on an activated NTA sensor chip in the measuring cell and human Sec62C as a negative control in the reference cell. Increasing concentrations of human Sec63C (as indicated) were passed over the chip and were followed by running buffer. Association and dissociation kinetics were recorded.
Figure 3.
Ribosome binding of human Sec62N and inhibition by basic oligopeptides. (A–C) Sec62N was adjusted to KCl concentration of 100 mM and incubated without (B) or with ribosomes (C). A third mixture contained ribosomes but was free of Sec62N (A). Subsequently the samples were subjected to sucrose gradient centrifugation. After fractionation of the gradients, aliquots of the fractions and the pellets (p) were subjected to SDS-PAGE and protein staining. (D–F) Recombinant proteins were incubated in the presence (D and E) or absence (F) of nontranslating ribosomes. Subsequently, the mixtures were layered onto a sucrose cushion and subjected to ultracentrifugation. The pellets (p) and supernatants (s) were analyzed by SDS-PAGE and subsequent protein staining (D) and Western blotting plus immunodetection with anti-penta-histidine antibodies (E and F). (G) Sec62N (150 pmol) or Sec62N in combination with the indicated oligopeptide (50 nmol) were incubated in the presence of nontranslating ribosomes. Subsequently, the mixtures were layered onto a sucrose cushion and subjected to ultracentrifugation. The pellets and supernatants were analyzed by SDS-PAGE and subsequent protein staining. (H–J) Recombinant proteins were incubated in the presence (H and I) or absence (J) of nontranslating ribosomes. Subsequently, the mixtures were analyzed as in D–F. We note that the recombinant proteins did not pellet in the absence of ribosomes (F and J). yeast, yeast Sec62N; h-yeast, humanized yeast Sec62N.
Figure 4.
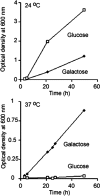
Human Sec62 complements a corresponding yeast mutant strain. Yeast SEC62 ts mutant strain RDM50-94C was grown at 24 or 37°C in the absence of uracil and in the presence of glucose or galactose as indicated. The cells had been transformed with a multicopy vector that contained URA3 as a selection marker and the human SEC62 cDNA under control of the GAL1 promoter. The optical density (OD) was measured at 600 nm.
Figure 5.
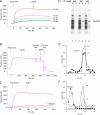
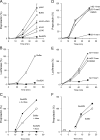
Characterization of the Sec62/ribosome interaction. (A) Human Sec62N was immobilized on an activated NTA sensor chip in the measuring cell and yeast Sec62N as a negative control in the reference cell. Increasing concentrations of nontranslating ribosomes (as indicated) were passed over the chip and were followed by running buffer. Association and dissociation kinetics were recorded. (B) Sensorgram after application of ribosomes, running buffer, and subsequent application of running buffer with 200 and 500 mM KOAc, respectively. (C) Sensorgram after application of ribosomes and the same concentration of ribosomes that had been pretreated with RNase A (240 μg/ml), respectively, for 30 min at 30°C. (D) Sec62N was incubated in the presence of nontranslating ribosomes at the indicated salt concentrations. Subsequently, the mixture was layered onto a sucrose cushion and subjected to ultracentrifugation. The pellets and supernatants were analyzed by SDS-PAGE and subsequent protein staining. (E and F) Sec62N was incubated in the presence of ribosomes that had been pretreated with buffer (E) or RNase A in buffer (80 μg/ml; F) for 30 min at 30°C. Subsequently the samples were subjected to sucrose gradient centrifugation. The fractions were analyzed by SDS-PAGE and subsequent Western blotting plus immunodetection with anti-penta-histidine (■), anti-L4 (◇), and anti-S3 (○) antibodies. The Western blot signals were quantified by luminescence imaging and plotted against the fraction number.
Figure 6.
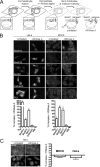
Sec62N binds near the ribosomal tunnel exit. (A–C) Sec62N was incubated in the absence (A) or presence of 60S (B) or 40S (C) ribosomal subunits. Subsequently the samples were subjected to sucrose gradient centrifugation. The fractions were analyzed by SDS-PAGE and subsequent Western blotting plus immunodetection with anti-penta-histidine (■), anti-L4 (◇), and anti-S3 (○) antibodies. The Western blot signals were quantified by luminescence imaging and plotted against the fraction number. (D) Human Sec62N was immobilized on an activated NTA sensor chip in the measuring cell and yeast Sec62N as a negative control in the reference cell. SPR sensorgram after application of ribosomes that had been preincubated with the indicated concentrations of Sec62N, ERj1C, or BSA for 10 min at 30°C. (E and F) ppl86mer was synthesized in reticulocyte lysate. The ribosomes were pelleted by ultracentrifugation and supplemented with buffer or recombinant protein as indicated (ERj1 served as a positive control; Dudek et al., 2005). After incubation and reisolation of ribosomes the cross-linking reagent was added, and the incubation was continued. All samples were subjected to SDS-PAGE. The positions of cross-linking products between ppl86mer and recombinant proteins are indicated. We note that different types of translation kit were used in the two experiments, which may account for the differences in cross-linking efficiencies.
Figure 7.
Sec62N but not Sec62N-ΔN10-ΔC40 is displaced from Sec63C by ribosomes. GST-Sec63C was immobilized on GSH-Sepharose. Sec62N (lanes 2–5) or Sec62N-ΔN10-ΔC40 (lanes 7–10) were applied to the resin in the presence of BSA. After washing, the resin was eluted with phosphate buffer (3 mM KCl, 1 mM MgCl2, 155 mM NaCl, 50 mM NaH2PO4, 200 mM Na2HPO4, pH 7.3, 0.65% CHAPS, 10 μg/ml BSA) or nontranslating ribosomes in phosphate buffer. After washing, the bound material was eluted and analyzed by SDS-PAGE followed by either protein staining (A) or Western blotting plus immunodetection with anti-penta-histidine antibodies (B).
Figure 8.
Inhibition of in vitro protein synthesis by Sec62N and by basic oligopeptides. (A and B) Preprolactin (A) or luciferase (B) were synthesized in reticulocyte lysate in the presence of [35S]methionine. The translation reactions were supplemented with buffer or recombinant protein (1 μM) in the same buffer. After the indicated incubation times, aliquots of the complete translation reactions were subjected to SDS-PAGE and phosphorimaging. The amount of preprolactin or luciferase that was present at the end of the buffer control reaction was set to 100%. Average values and SEMs are presented in A and were based on n = 6 (yeast) and n = 9 (all others). (C) Before translation, the reticulocyte lysate was fractionated into ribosomes and postribosomal supernatant by centrifugation (Dudek et al., 2002). Subsequently, the postribosomal supernatant was combined with the ribosomal pellet that corresponded to the same (□ and ■) or the fourfold (•) volume of reticulocyte lysate. The preprolactin was synthesized in the presence of buffer (□) or recombinant Sec62N (• and ■) and analyzed as described in A. (D and E) Preprolactin (D) or luciferase (E) were synthesized in reticulocyte lysate in the presence of [35S]methionine. The translation reactions were supplemented with DMSO or the indicated oligopeptide (150 μM) in DMSO and analyzed as described in A. (F) Synthesis of preprolactin was initiated for 6 min. Then aurintricarboxylic acid (ATA; 77 μM) was added in order to block further initiation (indicated by arrowhead), and the translation reactions were supplemented with buffer or recombinant protein in buffer and analyzed as described in A.
Figure 9.
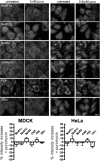
Sec62 is associated with ribosomes at the ER of permeabilized mammalian cells. (A) Experimental strategy. (B) HeLa and MDCK cells were fixed, left untreated or treated with RNase, and labeled with the indicated primary antibodies plus Alexa 555–conjugated secondary antibodies as described in Material and Methods. Fluorescence was recorded and quantified as described in Material and Methods. The difference in fluorescence intensity between the RNase-treated and the control sample is given as % change, and the SEM is indicated. Asterisk indicates significance as determined by Student's t test, i.e., p < 0.0001 relative to control (n > 20 cells). (C) HeLa and MDCK cells were fixed, left untreated or treated with RNase A, and labeled with the primary antibodies that were directed against SRa plus Alexa 555–conjugated secondary antibodies. We note that the axis scales are different for B versus C. Scale bar, 20 μm.
Figure 10.
Sec62 is associated with nontranslating ribosomes at the ER of permeabilized mammalian cells. HeLa and MDCK cells were incubated in the absence or presence of the indicated concentrations of puromycin for 1 h at 37°C. Then the cells were fixed and labeled with the indicated primary antibodies plus Alexa 555–conjugated secondary antibodies. We note that 1 mM puromycin is sufficient to inhibit translation within 5 min in MDCK cells and that the axis scales are different compared with Figure 9B. Scale bar, 20 μm.
Similar articles
- Emerging View on the Molecular Functions of Sec62 and Sec63 in Protein Translocation.
Jung SJ, Kim H. Jung SJ, et al. Int J Mol Sci. 2021 Nov 25;22(23):12757. doi: 10.3390/ijms222312757. Int J Mol Sci. 2021. PMID: 34884562 Free PMC article. Review. - Hph1 and Hph2 are novel components of the Sec63/Sec62 posttranslational translocation complex that aid in vacuolar proton ATPase biogenesis.
Piña FJ, O'Donnell AF, Pagant S, Piao HL, Miller JP, Fields S, Miller EA, Cyert MS. Piña FJ, et al. Eukaryot Cell. 2011 Jan;10(1):63-71. doi: 10.1128/EC.00241-10. Epub 2010 Nov 19. Eukaryot Cell. 2011. PMID: 21097665 Free PMC article. - Identification of signal peptide features for substrate specificity in human Sec62/Sec63-dependent ER protein import.
Schorr S, Nguyen D, Haßdenteufel S, Nagaraj N, Cavalié A, Greiner M, Weissgerber P, Loi M, Paton AW, Paton JC, Molinari M, Förster F, Dudek J, Lang S, Helms V, Zimmermann R. Schorr S, et al. FEBS J. 2020 Nov;287(21):4612-4640. doi: 10.1111/febs.15274. Epub 2020 Mar 20. FEBS J. 2020. PMID: 32133789 - Cotranslational Targeting and Posttranslational Translocation can Cooperate in Spc3 Topogenesis.
Jung SJ, Kim JEH, Junne T, Spiess M, Kim H. Jung SJ, et al. J Mol Biol. 2021 Sep 3;433(18):167109. doi: 10.1016/j.jmb.2021.167109. Epub 2021 Jun 18. J Mol Biol. 2021. PMID: 34153287 - The protein translocation apparatus of the rough endoplasmic reticulum, its associated proteins, and the mechanism of translocation.
Gilmore R. Gilmore R. Curr Opin Cell Biol. 1991 Aug;3(4):580-4. doi: 10.1016/0955-0674(91)90026-u. Curr Opin Cell Biol. 1991. PMID: 1772653 Review.
Cited by
- Alu RNA regulates the cellular pool of active ribosomes by targeted delivery of SRP9/14 to 40S subunits.
Ivanova E, Berger A, Scherrer A, Alkalaeva E, Strub K. Ivanova E, et al. Nucleic Acids Res. 2015 Mar 11;43(5):2874-87. doi: 10.1093/nar/gkv048. Epub 2015 Feb 19. Nucleic Acids Res. 2015. PMID: 25697503 Free PMC article. - Expression of 3q oncogene SEC62 in atypical fibroxanthoma-immunohistochemical analysis of 41 cases and correlation with clinical, viral and histopathologic features.
Müller CSL, Kreie L, Bochen F, Pfuhl T, Smola S, Gräber S, Vogt T, Schick B, Linxweiler M. Müller CSL, et al. Oncol Lett. 2019 Feb;17(2):1768-1776. doi: 10.3892/ol.2018.9767. Epub 2018 Nov 27. Oncol Lett. 2019. PMID: 30675236 Free PMC article. - Inhibitors of protein translocation across membranes of the secretory pathway: novel antimicrobial and anticancer agents.
Van Puyenbroeck V, Vermeire K. Van Puyenbroeck V, et al. Cell Mol Life Sci. 2018 May;75(9):1541-1558. doi: 10.1007/s00018-017-2743-2. Epub 2018 Jan 5. Cell Mol Life Sci. 2018. PMID: 29305616 Free PMC article. Review. - Protein folding and quality control in the endoplasmic reticulum: Recent lessons from yeast and mammalian cell systems.
Brodsky JL, Skach WR. Brodsky JL, et al. Curr Opin Cell Biol. 2011 Aug;23(4):464-75. doi: 10.1016/j.ceb.2011.05.004. Epub 2011 Jun 12. Curr Opin Cell Biol. 2011. PMID: 21664808 Free PMC article. Review. - BiP modulates the affinity of its co-chaperone ERj1 for ribosomes.
Benedix J, Lajoie P, Jaiswal H, Burgard C, Greiner M, Zimmermann R, Rospert S, Snapp EL, Dudek J. Benedix J, et al. J Biol Chem. 2010 Nov 19;285(47):36427-33. doi: 10.1074/jbc.M110.143263. Epub 2010 Sep 23. J Biol Chem. 2010. PMID: 20864538 Free PMC article.
References
- Beckmann R., Spahn C.M.T., Eswar N., Helmers J., Penczek P., Sali A., Frank J., Blobel G. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell. 2001;107:361–372. - PubMed
- Blau M., Mullapudi S., Becker T., Dudek J., Zimmermann R., Penczek P., Beckmann R. ERj1p uses a universal ribosomal adaptor site to coordinate the 80S ribosome at the membrane. Nat. Struct. Mol. Biol. 2005;12:1015–1016. - PubMed
- Davila S., et al. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat. Genet. 2004;36:575–577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM086530/GM/NIGMS NIH HHS/United States
- R01 GM086530-01/GM/NIGMS NIH HHS/United States
- R21 DK074650/DK/NIDDK NIH HHS/United States
- R21 DK074650-01/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases