Histone deacetylases as targets for the treatment of human neurodegenerative diseases - PubMed (original) (raw)
Review
Histone deacetylases as targets for the treatment of human neurodegenerative diseases
Santosh R D'Mello. Drug News Perspect. 2009 Nov.
Abstract
Histone deacetylases (HDACs) are a family of proteins that play an important role in regulating transcription as well as the function of a variety of cellular proteins. While these proteins are expressed abundantly in the brain, little is known about their roles in brain function. A growing body of evidence suggests that HDACs regulate neuronal survival. Results from studies conducted in vertebrate and mammalian experimental systems indicate that while some of these proteins are involved in promoting neuronal death, a majority of the HDACs studied thus far protect against neurodegeneration. Here we review the research performed on the role played by individual members of the HDAC family in the regulation of neuronal death. Chemical inhibitors of HDACs have been used in a variety of models of neurodegenerative disorders. We summarize the results from these studies, which indicate that HDAC inhibitors show great promise as therapeutic agents for human neurodegenerative disorders.
Copyright 2009 Prous Science, S.A.U. or its licensors. All rights reserved.
Figures
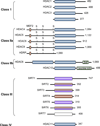
Figure 1
The histone deacetylase (HDAC) family of proteins. The conserved deacetylase domain of the classical HDACs (blue rectangles), deacetylase domain of the sirtuins (purple rectangles) and the ADP-ribosyl transferase catalytic domain (red rectangles) are indicated. Histone deacetylase-related protein (HDRP), also referred to as MITR, is a truncated form of HDAC9, generated by alternative splicing. SIRT2, SIRT3 and SIRT6 have both deacetylase and ADP-ribosyl transferase activities. No catalytic activity has been reported for SIRT7. The myocyte-specific enhancer factor 2A (MEF2)-binding site is marked by a blue square and the location of key serine residues that are phosphorylated, is indicated with “S”. The leucine-rich region in HDAC10 and the _C_-terminal zinc finger (ZnF) are also shown.
Similar articles
- Multiple roles of HDAC inhibition in neurodegenerative conditions.
Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Chuang DM, et al. Trends Neurosci. 2009 Nov;32(11):591-601. doi: 10.1016/j.tins.2009.06.002. Epub 2009 Sep 21. Trends Neurosci. 2009. PMID: 19775759 Free PMC article. Review. - HDAC inhibitors and neurodegeneration: at the edge between protection and damage.
Dietz KC, Casaccia P. Dietz KC, et al. Pharmacol Res. 2010 Jul;62(1):11-7. doi: 10.1016/j.phrs.2010.01.011. Epub 2010 Feb 1. Pharmacol Res. 2010. PMID: 20123018 Free PMC article. Review. - Histone Deacetylase Inhibitors: A Therapeutic Key in Neurological Disorders?
Ziemka-Nalecz M, Jaworska J, Sypecka J, Zalewska T. Ziemka-Nalecz M, et al. J Neuropathol Exp Neurol. 2018 Oct 1;77(10):855-870. doi: 10.1093/jnen/nly073. J Neuropathol Exp Neurol. 2018. PMID: 30165682 Review. - HDAC6 α-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases.
Li G, Jiang H, Chang M, Xie H, Hu L. Li G, et al. J Neurol Sci. 2011 May 15;304(1-2):1-8. doi: 10.1016/j.jns.2011.02.017. Epub 2011 Mar 5. J Neurol Sci. 2011. PMID: 21377170 Review. - HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis.
Saha RN, Pahan K. Saha RN, et al. Cell Death Differ. 2006 Apr;13(4):539-50. doi: 10.1038/sj.cdd.4401769. Cell Death Differ. 2006. PMID: 16167067 Free PMC article. Review.
Cited by
- Molecular Neuroprotection Induced by Zinc-Dependent Expression of Hepatitis C-Derived Protein NS5A Targeting Kv2.1 Potassium Channels.
Justice JA, Manjooran DT, Yeh CY, Hartnett-Scott KA, Schulien AJ, Kosobucki GJ, Mammen S, Palladino MJ, Aizenman E. Justice JA, et al. J Pharmacol Exp Ther. 2018 Nov;367(2):348-355. doi: 10.1124/jpet.118.252338. Epub 2018 Sep 6. J Pharmacol Exp Ther. 2018. PMID: 30190339 Free PMC article. - Effects of alcohol on histone deacetylase 2 (HDAC2) and the neuroprotective role of trichostatin A (TSA).
Agudelo M, Gandhi N, Saiyed Z, Pichili V, Thangavel S, Khatavkar P, Yndart-Arias A, Nair M. Agudelo M, et al. Alcohol Clin Exp Res. 2011 Aug;35(8):1550-6. doi: 10.1111/j.1530-0277.2011.01492.x. Epub 2011 Mar 29. Alcohol Clin Exp Res. 2011. PMID: 21447001 Free PMC article. - Histone hyperacetylation up-regulates protein kinase Cδ in dopaminergic neurons to induce cell death: relevance to epigenetic mechanisms of neurodegeneration in Parkinson disease.
Jin H, Kanthasamy A, Harischandra DS, Kondru N, Ghosh A, Panicker N, Anantharam V, Rana A, Kanthasamy AG. Jin H, et al. J Biol Chem. 2014 Dec 12;289(50):34743-67. doi: 10.1074/jbc.M114.576702. Epub 2014 Oct 23. J Biol Chem. 2014. PMID: 25342743 Free PMC article. - Improved HDAC Inhibition, Stronger Cytotoxic Effect and Higher Selectivity against Leukemias and Lymphomas of Novel, Tricyclic Vorinostat Analogues.
Bieszczad B, Garbicz D, Świtalska M, Dudek MK, Warszycki D, Wietrzyk J, Grzesiuk E, Mieczkowski A. Bieszczad B, et al. Pharmaceuticals (Basel). 2021 Aug 26;14(9):851. doi: 10.3390/ph14090851. Pharmaceuticals (Basel). 2021. PMID: 34577551 Free PMC article. - Epigenetic mechanisms in memory and synaptic function.
Sultan FA, Day JJ. Sultan FA, et al. Epigenomics. 2011 Apr;3(2):157-81. doi: 10.2217/epi.11.6. Epigenomics. 2011. PMID: 22122279 Free PMC article. Review.
References
- Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;2(59):177–189. - PubMed
- Wilson AJ, Byun DS, Popova N, Murray LB, L'Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J. Biol. Chem. 2006;19(281):13548–13558. - PubMed
- Zhang Z, Yamashita H, Toyama T, Sugiura H, Ando Y, Mita K, Hamaguchi M, Hara Y, Kobayashi S, Iwase H. Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast*. Breast Cancer Res. Treat. 2005;1(94):11–16. - PubMed
- Zhang Z, Yamashita H, Toyama T, Sugiura H, Omoto Y, Ando Y, Mita K, Hamaguchi M, Hayashi S, Iwase H. HDAC6 expression is correlated with better survival in breast cancer. Clin. Cancer Res. 2004;20(10):6962–6968. - PubMed
- Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;2(280):168–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical