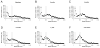Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus - PubMed (original) (raw)
Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus
Adeline Jabès et al. Eur J Neurosci. 2010 Jan.
Abstract
The dentate gyrus is one of only two regions of the mammalian brain where substantial neurogenesis occurs postnatally. However, detailed quantitative information about the postnatal structural maturation of the primate dentate gyrus is meager. We performed design-based, stereological studies of neuron number and size, and volume of the dentate gyrus layers in rhesus macaque monkeys (Macaca mulatta) of different postnatal ages. We found that about 40% of the total number of granule cells observed in mature 5-10-year-old macaque monkeys are added to the granule cell layer postnatally; 25% of these neurons are added within the first three postnatal months. Accordingly, cell proliferation and neurogenesis within the dentate gyrus peak within the first 3 months after birth and remain at an intermediate level between 3 months and at least 1 year of age. Although granule cell bodies undergo their largest increase in size during the first year of life, cell size and the volume of the three layers of the dentate gyrus (i.e. the molecular, granule cell and polymorphic layers) continue to increase beyond 1 year of age. Moreover, the different layers of the dentate gyrus exhibit distinct volumetric changes during postnatal development. Finally, we observe significant levels of cell proliferation, neurogenesis and cell death in the context of an overall stable number of granule cells in mature 5-10-year-old monkeys. These data identify an extended developmental period during which neurogenesis might be modulated to significantly impact the structure and function of the dentate gyrus in adulthood.
Figures
Figure 1
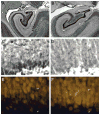
Coronal Nissl-stained sections at mid rostrocaudal level through the dentate gyrus of a newborn (A) and 7.7-year-old (B) macaque monkey (Macaca mulatta). ml: molecular layer; gcl: granule cell layer; pl: polymorphic layer. Scale bar: 500 μm in A, applies to panels A and B. C, Nissl-stained section illustrating the presence of a large population of immature neurons in the deep portion of the granule cell layer of a newborn monkey. D, Nissl-stained sections showing the same area in an adult monkey. Note the absence of the large population of immature neurons in the adult as compared to the newborn monkey. E, NeuN-immunostained section showing that the large population of cells identified as immature granule cells in Nissl preparations (C) are consistently, but faintly labeled with NeuN. F, NeuN-immunostained section showing the same area in an adult monkey. All neurons in the adult granule cell layer are heavily labeled with NeuN. Scale bar: 10 μm in C, applies to panels C, D, E and F.
Figure 2
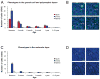
Ki-67-immunohistochemistry. A, Number of Ki-67-positive cells in the molecular layer (white diamonds) and in the combined granule cell and polymorphic layers (black circles) of the macaque monkey dentate gyrus through early postnatal development and in adulthood. Note the intermediate level of cell proliferation between three months and one year of age. B, Photomicrograph of Ki-67-labeled cells in the granule cell and polymorphic layers of the dentate gyrus. Scale bar: 10 μm. Abbreviations, see Fig. 1.
Figure 3
Phenotype of BrdU-labeled cells in the macaque monkey dentate gyrus, four weeks following BrdU injection (150 mg/kg). A, Phenotype of BrdU-labeled cells in the combined granule cell and polymorphic layers. Note the significant rate of Brdu/NeuN-positive cells until one year of age. B, Confocal image of BrdU/NeuN-positive (yellow arrow) and BrdU/S100beta-positive (white arrowheads) cells at the border between the granule cell and polymorphic layers. Scale bar: 10 μm, applies to all panels. C, Phenotype of BrdU-labeled cells in the molecular layer. Note the absence of BrdU/NeuN-positive cells in the molecular layer. D, Confocal image of a BrdU/S100beta-positive cell (white arrowhead) in the molecular layer. The different panels in B and D represent different planes of section (1 μm apart) through the depth of the labeled cells. Scale bar: 10 μm, applies to all panels.
Figure 4
Representative coronal sections through the monkey dentate gyrus illustrating the location of Ki-67-positive cells (A–C), BrdU-positive cells four weeks after BrdU Injection (D–F) and TUNEL-positive cells (G–I), at different ages through postnatal life (A,D,G: newborns; B,E,H: 1-year-olds; C,F,I: 5–10-year-olds). Note the different distribution of Ki-67- and BrdU-labeled cells across the polymorphic and granule cell layers. Note also the location of TUNEL-positive cells across all layers of the dentate gyrus, including the granule cell layer. Scale bar: 500 μm in I, applies to all panels. Abbreviations, see Fig. 1.
Figure 5
TUNEL staining. A, Number of TUNEL-positive cells in the molecular layer (white diamonds) and in the combined granule cell and polymorphic layers (black circles) of the macaque monkey dentate gyrus through early postnatal development and in adulthood. B, Photomicrograph of a TUNEL-positive cell in the granule cell layer. Scale bar: 10 μm.
Figure 6
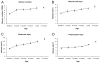
Total number of neurons in the granule cell layer (A) and volumes (B,C,D) of the different layers of the macaque monkey dentate gyrus through early postnatal development and in adulthood. Note the large increase in neuron number between birth and three months of age, and the gradual increase of about 3,100 neurons per day between three months and one year of age. B, Volume of the granule cell layer. C, Volume of the molecular layer. D, Volume of the polymorphic layer. Note the linear increase in volume of the granule cell and molecular layers between birth and one year of age. In contrast, the polymorphic layer exhibits a significant increase only past nine months and one year of age.
Figure 7
Bimodal distribution of neuronal soma size in the monkey granule cell layer through early postnatal development and in adulthood. Grey areas indicate the two modes: mode 1, corresponding to immature granule cells (soma < 150 μm3); mode 2, corresponding to the median size of mature granule cells (400–550μm3). A, newborns. B, 3-month-olds. C, 6-month-olds. D, 9-month-olds. E, 1-year-olds. F, 5–10-year-olds.
Similar articles
- Postnatal neurogenesis in the dentate gyrus of the guinea pig.
Guidi S, Ciani E, Severi S, Contestabile A, Bartesaghi R. Guidi S, et al. Hippocampus. 2005;15(3):285-301. doi: 10.1002/hipo.20050. Hippocampus. 2005. PMID: 15515010 - A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis.
Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH. Mathews EA, et al. J Comp Neurol. 2010 Nov 15;518(22):4479-90. doi: 10.1002/cne.22489. J Comp Neurol. 2010. PMID: 20886617 Free PMC article. - A morphologically distinct granule cell type in the dentate gyrus of the red fox correlates with adult hippocampal neurogenesis.
Amrein I, Slomianka L. Amrein I, et al. Brain Res. 2010 Apr 30;1328:12-24. doi: 10.1016/j.brainres.2010.02.075. Epub 2010 Mar 4. Brain Res. 2010. PMID: 20206610 - Morphological development and maturation of granule neuron dendrites in the rat dentate gyrus.
Rahimi O, Claiborne BJ. Rahimi O, et al. Prog Brain Res. 2007;163:167-81. doi: 10.1016/S0079-6123(07)63010-6. Prog Brain Res. 2007. PMID: 17765718 Review. - Adult neurogenesis in the mammalian dentate gyrus.
Abbott LC, Nigussie F. Abbott LC, et al. Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review.
Cited by
- Cell age-specific vulnerability of neurons to anesthetic toxicity.
Hofacer RD, Deng M, Ward CG, Joseph B, Hughes EA, Jiang C, Danzer SC, Loepke AW. Hofacer RD, et al. Ann Neurol. 2013 Jun;73(6):695-704. doi: 10.1002/ana.23892. Epub 2013 Jun 5. Ann Neurol. 2013. PMID: 23526697 Free PMC article. - Postnatal development of the entorhinal cortex: A stereological study in macaque monkeys.
Piguet O, J Chareyron L, Banta Lavenex P, G Amaral D, Lavenex P. Piguet O, et al. J Comp Neurol. 2020 Oct;528(14):2308-2332. doi: 10.1002/cne.24897. Epub 2020 Mar 18. J Comp Neurol. 2020. PMID: 32134112 Free PMC article. - Comparing Adult Hippocampal Neurogenesis Across Species: Translating Time to Predict the Tempo in Humans.
Charvet CJ, Finlay BL. Charvet CJ, et al. Front Neurosci. 2018 Oct 5;12:706. doi: 10.3389/fnins.2018.00706. eCollection 2018. Front Neurosci. 2018. PMID: 30344473 Free PMC article. - Hippocampal functional connectivity and episodic memory in early childhood.
Riggins T, Geng F, Blankenship SL, Redcay E. Riggins T, et al. Dev Cogn Neurosci. 2016 Jun;19:58-69. doi: 10.1016/j.dcn.2016.02.002. Epub 2016 Feb 9. Dev Cogn Neurosci. 2016. PMID: 26900967 Free PMC article. - Dynamics of hippocampal neurogenesis in adult humans.
Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J. Spalding KL, et al. Cell. 2013 Jun 6;153(6):1219-1227. doi: 10.1016/j.cell.2013.05.002. Cell. 2013. PMID: 23746839 Free PMC article.
References
- Altemus KL, Lavenex P, Ishizuka N, Amaral DG. Morphological characteristics and electrophysiological properties of CA1 pyramidal neurons in macaque monkeys. Neuroscience. 2005;136:741–756. - PubMed
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Amaral DG, Lavenex P. Hippocampal neuroanatomy. In: Amaral DG, Andersen P, Bliss T, Morris RGM, O’Keefe J, editors. The Hippocampus Book. New York: Oxford University Press; 2007. pp. 37–114.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS016980-28/NS/NINDS NIH HHS/United States
- P51 RR000169/RR/NCRR NIH HHS/United States
- R01-NS16980/NS/NINDS NIH HHS/United States
- R01 NS016980/NS/NINDS NIH HHS/United States
- RR00169/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources