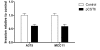Cystatin E/M suppresses legumain activity and invasion of human melanoma - PubMed (original) (raw)
Cystatin E/M suppresses legumain activity and invasion of human melanoma
Jon J Briggs et al. BMC Cancer. 2010.
Abstract
Background: High activity of cysteine proteases such as legumain and the cathepsins have been shown to facilitate growth and invasion of a variety of tumor types. In breast cancer, several recent studies have indicated that loss of the cysteine protease inhibitor cystatin E/M leads to increased growth and metastasis. Although cystatin E/M is normally expressed in the skin, its role in cysteine protease regulation and progression of malignant melanoma has not been studied.
Methods: A panel of various non-melanoma and melanoma cell lines was used. Cystatin E/M and C were analyzed in cell media by immunoblotting and ELISA. Legumain, cathepsin B and L were analyzed in cell lysates by immunoblotting and their enzymatic activities were analyzed by peptide substrates. Two melanoma cell lines lacking detectable secretion of cystatin E/M were transfected with a cystatin E/M expression plasmid (pCST6), and migration and invasiveness were studied by a Matrigel invasion assay.
Results: Cystatin E/M was undetectable in media from all established melanoma cell lines examined, whereas strong immunobands were detected in two of five primary melanoma lines and in two of six lines derived from patients with metastatic disease. Among the four melanoma lines secreting cystatin E/M, the glycosylated form (17 kD) was predominant compared to the non-glycosylated form (14 kD). Legumain, cathepsin B and L were expressed and active in most of the cell lines, although at low levels in the melanomas expressing cystatin E/M. In the melanoma lines where cystatin E/M was secreted, cystatin C was generally absent or expressed at a very low level. When melanoma cells lacking secretion of cystatin E/M were transfected with pCST6, their intracellular legumain activity was significantly inhibited. In contrast, cathepsin B activity was not affected. Furthermore, invasion was suppressed in cystatin E/M over-expressing melanoma cell lines as measured by the transwell Matrigel assay.
Conclusions: These results suggest that the level of cystatin E/M regulates legumain activity and hence the invasive potential of human melanoma cells.
Figures
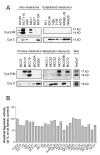
Figure 1
Cystatin E/M and C secretions from various cell lines: Common laboratory non-melanoma and established laboratory melanoma cell lines (top), and primary and metastatic melanoma cell lines established from patients, as well as skin control (bottom). (A) Equal amounts of serum free media were collected from 5 × 105 cells 48 h after changing from ordinary growth media and secreted proteins were concentrated by TCA-precipitation and subjected to SDS-PAGE and immunoblotting. The filters were stained with a cystatin E/M-specific (upper panels) or a cystatin C-specific (lower panel) antibody, respectively. (B) Inhibitory activity against legumain was measured in the conditioned media as residual legumain activity. A partially purified legumain fraction from rat kidney was mixed with conditioned media and the ability to cleave the substrate Z-Ala-Ala-Asn-NHMec was measured by fluorometry. Control bar (100%) represents activity in the rat legumain fraction without addition of conditioned media.
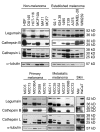
Figure 2
Legumain, cathepsin B and L expressions in various cell lines: Common laboratory non-melanoma and established laboratory melanoma cell lines (top), and primary and metastatic melanoma cell lines established from patients, as well as skin controls (bottom). Whole cell extracts were collected 24 h after seeding and equal amounts of total protein (25 μg) were used for SDS-PAGE and immunoblotting. The filters were stained with a legumain-specific (upper panels), cathepsin B- and L-specific (middle panels) or α-tubulin specific (lower panels) antibody, respectively.
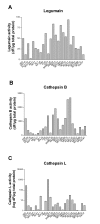
Figure 3
Enzyme activities of legumain (A), cathepsin B (B) and cathepsin L (C) in various cell lines. Whole cell extracts from common laboratory non-melanoma and established laboratory melanoma cell lines, primary and metastatic melanoma cell lines established from patients, and skin cell controls were collected 24 h after seeding. Legumain (A), cathepsin B (B) and L (C) activities in each cell lysate were measured by cleavage of peptide substrates and fluorometry (see Materials and methods for details). Note: The Y-axis in A and B are shown as dF/μg total protein whereas y-axis in C is shown as log dF/μg total protein.
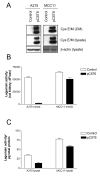
Figure 4
Cystatin E/M over-expression in melanoma cells suppressed legumain activity: A375 and MCC11 cells were transfected with pCST6 (closed bars) or empty pTracer vector (control; open bars), media were changed the following day, and serum-free conditioned media and cell lysates were collected 24 h later. (A) Immunoblots of cystatin E/M in conditioned media (CM) and lysates. Secreted proteins were TCA-precipitated prior to SDS-PAGE and immunoblotting. The filters were stained with a cystatin E/M-specific (upper and middle panels) or a β-actin specific (lower panel; loading control) antibody, respectively. (B) Inhibitory activity against legumain was measured in the conditioned media as residual legumain activity using fluorometry, a fixed amount of partially purified legumain from rat kidney and the substrate Z-Ala-Ala-Asn-NHMec (A375, p < 0.0001; MCC11, p = 0.002). (C) Activity of legumain in cell lysates measured by cleavage of Z-Ala-Ala-Asn-NHMec (A375, p < 0.0001; MCC11, p = 0.0012). Error bars represent the standard error of the mean (SEM).
Figure 5
Over-expression of cystatin E/M suppressed melanoma cell invasion. A375 and MCC11 cells were transfected with pCST6 (closed bars) or empty vector (control; open bars) and migration through Matrigel coated transwell membranes was determined. The bars represent the average of four or three experiments for A375 and MCC11, respectively, with six replicate wells for each transfection group (A375, p < 0.01; MCC11, p < 0.05). Error bars represent the standard error of the mean (SEM).
Similar articles
- Low-level internalization of cystatin E/M affects legumain activity and migration of melanoma cells.
Wallin H, Apelqvist J, Andersson F, Ekström U, Abrahamson M. Wallin H, et al. J Biol Chem. 2017 Sep 1;292(35):14413-14424. doi: 10.1074/jbc.M117.776138. Epub 2017 Jun 19. J Biol Chem. 2017. PMID: 28630039 Free PMC article. - Silencing of cystatin M in metastatic oral cancer cell line MDA-686Ln by siRNA increases cysteine proteinases and legumain activities, cell proliferation and in vitro invasion.
Vigneswaran N, Wu J, Nagaraj N, James R, Zeeuwen P, Zacharias W. Vigneswaran N, et al. Life Sci. 2006 Jan 18;78(8):898-907. doi: 10.1016/j.lfs.2005.05.096. Epub 2005 Sep 8. Life Sci. 2006. PMID: 16150465 - Intra- and extracellular regulation of activity and processing of legumain by cystatin E/M.
Smith R, Johansen HT, Nilsen H, Haugen MH, Pettersen SJ, Mælandsmo GM, Abrahamson M, Solberg R. Smith R, et al. Biochimie. 2012 Dec;94(12):2590-9. doi: 10.1016/j.biochi.2012.07.026. Epub 2012 Aug 10. Biochimie. 2012. PMID: 22902879 - The biology of cystatin M/E and its cognate target proteases.
Zeeuwen PL, Cheng T, Schalkwijk J. Zeeuwen PL, et al. J Invest Dermatol. 2009 Jun;129(6):1327-38. doi: 10.1038/jid.2009.40. Epub 2009 Mar 5. J Invest Dermatol. 2009. PMID: 19262604 Review. - Cystatin M/E (Cystatin 6): A Janus-Faced Cysteine Protease Inhibitor with Both Tumor-Suppressing and Tumor-Promoting Functions.
Lalmanach G, Kasabova-Arjomand M, Lecaille F, Saidi A. Lalmanach G, et al. Cancers (Basel). 2021 Apr 14;13(8):1877. doi: 10.3390/cancers13081877. Cancers (Basel). 2021. PMID: 33919854 Free PMC article. Review.
Cited by
- A multifunctional protease inhibitor to regulate endolysosomal function.
van Kasteren SI, Berlin I, Colbert JD, Keane D, Ovaa H, Watts C. van Kasteren SI, et al. ACS Chem Biol. 2011 Nov 18;6(11):1198-204. doi: 10.1021/cb200292c. Epub 2011 Sep 19. ACS Chem Biol. 2011. PMID: 21910425 Free PMC article. - Clinicopathologic significance of legumain overexpression in cancer: a systematic review and meta-analysis.
Zhen Y, Chunlei G, Wenzhi S, Shuangtao Z, Na L, Rongrong W, Xiaohe L, Haiying N, Dehong L, Shan J, Xiaoyue T, Rong X. Zhen Y, et al. Sci Rep. 2015 Nov 26;5:16599. doi: 10.1038/srep16599. Sci Rep. 2015. PMID: 26607955 Free PMC article. Review. - Counter Selection Substrate Library Strategy for Developing Specific Protease Substrates and Probes.
Poreba M, Solberg R, Rut W, Lunde NN, Kasperkiewicz P, Snipas SJ, Mihelic M, Turk D, Turk B, Salvesen GS, Drag M. Poreba M, et al. Cell Chem Biol. 2016 Aug 18;23(8):1023-35. doi: 10.1016/j.chembiol.2016.05.020. Epub 2016 Jul 28. Cell Chem Biol. 2016. PMID: 27478158 Free PMC article. - Proteolysis mediated by cysteine cathepsins and legumain-recent advances and cell biological challenges.
Brix K, McInnes J, Al-Hashimi A, Rehders M, Tamhane T, Haugen MH. Brix K, et al. Protoplasma. 2015 May;252(3):755-74. doi: 10.1007/s00709-014-0730-0. Epub 2014 Nov 16. Protoplasma. 2015. PMID: 25398648 Review. - Knockdown of Legumain Suppresses Cervical Cancer Cell Migration and Invasion.
Meng F, Liu W. Meng F, et al. Oncol Res. 2016 Jan 21;23(1-2):7-12. doi: 10.3727/096504015X14410238486649. Oncol Res. 2016. PMID: 26802645 Free PMC article.
References
- Abrahamson M, Alvarez-Fernandez M, Nathanson CM. Cystatins. Biochem Soc Symp. 2003;70:179–199. - PubMed
- Henskens YMC, Veerman ECI, Amerongen AVN. Cystatins in health and disease. Biol Chem Hoppe-Seyler. 1996;377(2):71–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical