Mucolipins: Intracellular TRPML1-3 channels - PubMed (original) (raw)
Review
Mucolipins: Intracellular TRPML1-3 channels
Xiping Cheng et al. FEBS Lett. 2010.
Abstract
The mucolipin family of Transient Receptor Potential (TRPML) proteins is predicted to encode ion channels expressed in intracellular endosomes and lysosomes. Loss-of-function mutations of human TRPML1 cause type IV mucolipidosis (ML4), a childhood neurodegenerative disease. Meanwhile, gain-of-function mutations in the mouse TRPML3 result in the varitint-waddler (Va) phenotype with hearing and pigmentation defects. The broad spectrum phenotypes of ML4 and Va appear to result from certain aspects of endosomal/lysosomal dysfunction. Lysosomes, traditionally believed to be the terminal "recycling center" for biological "garbage", are now known to play indispensable roles in intracellular signal transduction and membrane trafficking. Studies employing animal models and cell lines in which TRPML genes have been genetically disrupted or depleted have uncovered roles of TRPMLs in multiple cellular functions including membrane trafficking, signal transduction, and organellar ion homeostasis. Physiological assays of mammalian cell lines in which TRPMLs are heterologously overexpressed have revealed the channel properties of TRPMLs in mediating cation (Ca(2+)/Fe(2+)) efflux from endosomes and lysosomes in response to unidentified cellular cues. This review aims to summarize these recent advances in the TRPML field and to correlate the channel properties of endolysosomal TRPMLs with their biological functions. We will also discuss the potential cellular mechanisms by which TRPML deficiency leads to neurodegeneration.
Copyright 2010 Federation of European Biochemical Societies. All rights reserved.
Figures
Figure 1. TRPMLs in the endocytic pathway
Intracellular compartments undergo cargo-dependent maturation (indicated by black arrows), membrane fusion (white arrows), and fission/budding (red arrows). The molecular identities of intracellular compartments are defined by specific recruitment of small G proteins (Rab and Arf GTPases) and the composition of phosphoinositides (PIPs). Endolysosomes are Ca2+ stores with luminal Ca2+ concentration estimated to be approximately 0.5 mM. The pH of each organelle is indicated. In endolysosomes, TRPML-mediated intra-endosomal Ca2+ release may activate Ca2+ sensor proteins such as Synaptotagmin (Syt) and calmodulin (CaM) to trigger homotypic and heterotypic fusion. TRPML3 is present in early endosomes (EEs; pH 6.0; PI(3)P; Rab5), which are derived from the primary endocytic vesicles after endocytosis. In addition to the late endocytic pathway, contents in the EE can also be sorted into recycling endosomes (RE; pH 6.4), which are subsequently recycled back to the plasma membrane. TRPML2 is present in Arf6 – positive REs. The channel activity of TRPML2 (in RE) may regulate the activation of small GTPase Arf6, an important regulator of the recycling pathway. EEs can undergo maturation (membrane trafficking) to become late endosomes (LEs; pH 5.5; PI(3)P+PI(3,5)P2; Rab7). Membrane proteins enter the degradation pathway following membrane invagination to form multi-vesicular bodies (MVB) in LEs. LEs can fuse with LYs (LYs; pH 4.5; PI(3)P+PI(3,5)P2; Rab7) to form LE-LY hybrids. LYs can then be reformed from LE-LY hybrids. Other than fusion with LEs, LYs can also undergo fusion with autophagosomes (APs) to form autolysosomes (ALs) or with the plasma membrane during lysosomal exocytosis. TRPML1-3 channels are predominantly localized in LEs and LYs. Activation of TRPML channels by unidentified cellular cues may induce intralysosomal Ca2+ release. LEs, LYs, or hybrids of LEs and LYs will then undergo CaM- or Syt- dependent membrane fusion or fission/budding. Retrograde transport vesicles, derived from EEs, LEs, or LYs upon membrane fission transport lipids and proteins in a retrograde direction to the trans-Golgi Network (TGN).
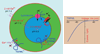
Figure 2. TRPMLs in the lysosome
A) While the ionic compositions of the extracellular space and cytosol have been well established, ion concentrations in the lumen of lysosomes are not clear. The luminal pH is between 4 and 5 and is established by a V-type ATPase. The [Ca2+]LY is approximately 0.5 mM and maintained by an unidentified H+-Ca2+ exchanger. The resting membrane potential (Δφ) of the cell is approximately −70 mV (cytoplasmic-side negative). Based on studies of phagosomes, the membrane potential across the lysosomal membrane is estimated to be approximately + 30 mV (luminal-side positive). TRPMLs are permeable to Fe2+ (except for TRPML3) and Ca2+; none of the TRPML isoforms are permeable to H+. Although wild-type TRPMLs are predominantly expressed in the lysosome, TRPML_Va_proteins are present at the plasma membrane. B) Current-voltage (I–V) relationship of TRPML channels. All TRPMLs exhibit a strong inward rectification.
Similar articles
- The role of TRPMLs in endolysosomal trafficking and function.
Venkatachalam K, Wong CO, Zhu MX. Venkatachalam K, et al. Cell Calcium. 2015 Jul;58(1):48-56. doi: 10.1016/j.ceca.2014.10.008. Epub 2014 Oct 28. Cell Calcium. 2015. PMID: 25465891 Free PMC article. Review. - The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel.
Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. Dong XP, et al. Nature. 2008 Oct 16;455(7215):992-6. doi: 10.1038/nature07311. Epub 2008 Sep 14. Nature. 2008. PMID: 18794901 Free PMC article. - Drosophila TRPML forms PI(3,5)P2-activated cation channels in both endolysosomes and plasma membrane.
Feng X, Huang Y, Lu Y, Xiong J, Wong CO, Yang P, Xia J, Chen D, Du G, Venkatachalam K, Xia X, Zhu MX. Feng X, et al. J Biol Chem. 2014 Feb 14;289(7):4262-72. doi: 10.1074/jbc.M113.506501. Epub 2013 Dec 27. J Biol Chem. 2014. PMID: 24375408 Free PMC article. - Heteromultimeric TRPML channel assemblies play a crucial role in the regulation of cell viability models and starvation-induced autophagy.
Zeevi DA, Lev S, Frumkin A, Minke B, Bach G. Zeevi DA, et al. J Cell Sci. 2010 Sep 15;123(Pt 18):3112-24. doi: 10.1242/jcs.067330. Epub 2010 Aug 24. J Cell Sci. 2010. PMID: 20736310 Free PMC article. - TRPML Cation Channels in Inflammation and Immunity.
Spix B, Chao YK, Abrahamian C, Chen CC, Grimm C. Spix B, et al. Front Immunol. 2020 Feb 28;11:225. doi: 10.3389/fimmu.2020.00225. eCollection 2020. Front Immunol. 2020. PMID: 32184778 Free PMC article. Review.
Cited by
- White matter abnormalities and iron deposition in prenatal mucolipidosis IV- fetal imaging and pathology.
Zerem A, Ben-Sira L, Vigdorovich N, Leibovitz Z, Fisher Y, Schiffmann R, Grishchuk Y, Misko AL, Orenstein N, Lev D, Lerman-Sagie T, Kidron D. Zerem A, et al. Metab Brain Dis. 2021 Oct;36(7):2155-2167. doi: 10.1007/s11011-021-00742-3. Epub 2021 May 8. Metab Brain Dis. 2021. PMID: 33963976 - Carboxyl-Terminal SSLKG Motif of the Human Cystinosin-LKG Plays an Important Role in Plasma Membrane Sorting.
Bellomo F, Taranta A, Petrini S, Venditti R, Rocchetti MT, Rega LR, Corallini S, Gesualdo L, De Matteis MA, Emma F. Bellomo F, et al. PLoS One. 2016 May 5;11(5):e0154805. doi: 10.1371/journal.pone.0154805. eCollection 2016. PLoS One. 2016. PMID: 27148969 Free PMC article. - Impaired autophagic flux and dedifferentiation in podocytes lacking Asah1 gene: Role of lysosomal TRPML1 channel.
Li G, Huang D, Zou Y, Kidd J, Gehr TWB, Li N, Ritter JK, Li PL. Li G, et al. Biochim Biophys Acta Mol Cell Res. 2023 Jan;1870(1):119386. doi: 10.1016/j.bbamcr.2022.119386. Epub 2022 Oct 24. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36302466 Free PMC article. - Transient Receptor Potential Mucolipin-1 Participates in Intracerebral Hemorrhage-Induced Secondary Brain Injury by Inducing Neuroinflammation and Neuronal Cell Death.
Shi J, Li X, Ding J, Lian J, Zhong Y, Li H, Shen H, You W, Fu X, Chen G. Shi J, et al. Neuromolecular Med. 2023 Jun;25(2):272-285. doi: 10.1007/s12017-023-08734-5. Epub 2023 Feb 4. Neuromolecular Med. 2023. PMID: 36737508 - Characterization of Endo-Lysosomal Cation Channels Using Calcium Imaging.
Wahl-Schott C, Freichel M, Hennis K, Philippaert K, Ottenheijm R, Tsvilovskyy V, Varbanov H. Wahl-Schott C, et al. Handb Exp Pharmacol. 2023;278:277-304. doi: 10.1007/164_2023_637. Handb Exp Pharmacol. 2023. PMID: 36894791
References
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. - PubMed
- Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Curr Opin Cell Biol. 2005;17:135–140. - PubMed
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. - PubMed
- Luzio JP, Bright NA, Pryor PR. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans. 2007;35:1088–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50-AG08671/AG/NIA NIH HHS/United States
- R01 NS062792/NS/NINDS NIH HHS/United States
- R01 NS062792-02/NS/NINDS NIH HHS/United States
- NS062792/NS/NINDS NIH HHS/United States
- P50 AG008671/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous