BRCA1 protein and nucleolin colocalize in breast carcinoma tissue and cancer cell lines - PubMed (original) (raw)
BRCA1 protein and nucleolin colocalize in breast carcinoma tissue and cancer cell lines
Natalie Tulchin et al. Am J Pathol. 2010 Mar.
Abstract
The breast and ovarian cancer susceptibility gene BRCA1 encodes a tumor suppressor. BRCA1 protein, which is involved in DNA damage response, has been thought to be found primarily in cell nuclei. In the present investigation, immunohistological studies of BRCA1 protein in frozen breast cancer tissue and MCF7 and HeLa cell lines revealed BRCA1 expression in both nucleoli and nucleoplasmic foci. Immunoelectron microscopic studies of estrogen-stimulated MCF7 cells demonstrated BRCA1 protein localization in the granular components of the nucleolus. Moreover, immunofluorescence of BRCA1 and nucleolin double-labeling showed colocalization in both nucleoli and nucleoplasmic foci in breast tumor cells and asynchronously growing MCF7 and HeLa cells. Multiparameter analysis of BRCA1 and nucleolin in relation to cell cycle position (DNA content) showed expression during G1-S and persistence of BRCA1 during G2/M. After gamma-irradiation of MCF7 cells, BRCA1 protein dispersed from nucleoli and nucleoplasmic foci to other nucleoplasmic sites, which did not colocalize with nucleolin. Small interfering RNA-mediated knockdown of BRCA1 protein resulted in decreased immunofluorescence staining, which was confirmed by Western blotting. The observed colocalization of BRCA1 and nucleolin raises new possibilities for the nucleoplasm-nucleolus pathways of these proteins and their functional significance.
Figures
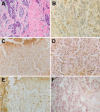
Figure 1
Comparison of mouse monoclonal BRCA1 antibodies: MS110, MS13, SG11, and AP16, and the rabbit polyclonal K-18 antibody in frozen tissue sections of a poorly differentiated adenocarcinoma from patient number 1. A: H&E-stained frozen section Original magnification ×270. B: Sequential section of the same tumor stained with MS110 mouse monoclonal BRCA1 antibody and anti-mouse IgG and avidin-biotin peroxidase detection system. Scattered tumor cell nuclei show BRCA1 staining Original magnification ×540. C: Sequential section stained with MS13 antibody shows scattered nuclear staining as well as some nonspecific staining of stromal material. Original magnification ×540. D: Sequential section stained with AP16 mouse monoclonal antibody shows focal nuclear staining. Original magnification ×540. E: Sequential section stained with SG11 antibody shows scattered tumor cell nuclear staining and nonspecific stromal staining. Original magnification ×540. F: Sequential section stained with the rabbit polyclonal K-18 antibody and anti-rabbit IgG and avidin-biotin peroxidase detection system shows nuclear staining of tumor cells. Original magnification ×540.
Figure 2
Nucleolar BRCA1 protein localization in MCF7 cells. MCF7 cells were treated (A, B) or not (C−F) with 10 nmol/L of 17 β-estradiol for 7 days. MCF7 cells were cultured and prepared as described in Materials and Methods, and immunostained with BRCA1 monoclonal antibody MS110 (Ab-1). A and B: Labeled nuclear dots for BRCA1 protein were observed in the nucleoplasm or in the periphery of the nucleoli (arrows), as it is better observed at higher magnification (B). C−F: In nontreated cells, labeled dots were mainly observed in the nucleoplasm, near nucleoli (C, arrows), as shown at higher magnification (D). Only some groups of few cells with labeled nuclei were observed at lower magnification (E) and no labeling was observed in cells incubated with the non relevant monoclonal antibody MOPC 21 (F). G: BRCA1 staining intensity quantified with an image analyzer in nuclei alone or nuclei plus cytoplasm of the same cells showed a significant increase in E2-treated cells’ nuclei (*P < 0.002) or with nuclei plus cytoplasm of E2 treated cells (**P < 0.0001). H: BRCA1 protein detected with MS110 antibody is significantly lower in whole cell lysates of control MCF7 cells compared with E2 treated cells. The histogram represents the BRCA1 concentration normalized with the reciprocal concentration of the α-tubulin (*P < 0.0001). I: In whole cell lysates of control MCF7 cells, the same results were observed with K-18 (C total a) and MS110 (C total b) primary antibodies. The presence of BRCA1 protein was observed also in cytoplasm and nuclear fractions of control cells at similar concentrations after protein normalization with MS110 antibody. α-tubulin and c-jun expression was used as a control for cytoplasm and nuclear fraction purity, respectively. Scale bars = 10 μm.
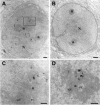
Figure 3
Nucleolar localization of BRCA1 protein using transmission electron microscopy. MCF7 cells were immunostained with BRCA1 monoclonal antibody MS110 (Ab-1) or non-relevant monoclonal antibody MOPC21. A: Labeled nuclear dots for BRCA1 protein were observed around nucleoli, sometimes in close contact with nucleolus fibers or within electron dense granular components (GC) of the nucleolar periphery. BRCA1-labeled protein was also observed in the cytoplasm, near the nuclear membrane in multivesicular bodies near the Golgi. The box plotted in A is represented at a higher magnification in C and the nucleolus of another cell at a high magnification in D. BRCA1 staining was detected in GCs of nucleoli surrounding the fibrillar center (FC). B: No labeled dots were observed in nuclei or nucleoli of cells incubated with the nonrelevant monoclonal antibody. N: nucleus, n: nucleolus. Scale bar = 0.25 μm.
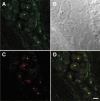
Figure 4
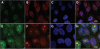
Colocalization of BRCA1 and nucleolin proteins in frozen tissue sections from poorly differentiated mammary adenocarcinoma from patient number 16. A: Rabbit polyclonal BRCA1 K-18 antibody stains many tumor cell nuclei (green). B: DIC Nomarski image of the same field as A, C, and D. C: Mouse monoclonal nucleolin antibody stains nucleoli (red). D: DIC image of C, nuclei stained for BRCA1 (green) merge with nucleolin (red) to show colocalization (yellow). There is also clumped BRCA1 staining (green). Scale bar = 10 μm.
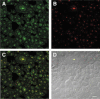
Figure 5
Colocalization of BRCA1 and nucleolin in an asynchronous culture of MCF7 cells. A: The rabbit polyclonal K-18 BRCA1 antibody stains many cell nuclei (green). B: Mouse monoclonal antibody to nucleolin stains nucleoli and many dot-like speckles (red). C: DIC image of B. Nuclei stain for BRCA1 (green) merge with nucleolin (red) to show colocalization (yellow). The merge occurs both in nucleoli (which are phase dense in D) and in some nuclear speckles. Not all of the nucleoli show BRCA1 colocalization, which provides an internal control for BRCA1 staining specificity. D: Nomarski image of the same field as A−C. Scale bar = 20 μm.
Figure 6
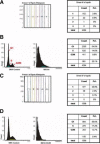
Detection of BRCA1 and nucleolin proteins during the cell cycle by LSC. For BRCA1 staining, MCF7 cells were indirectly stained with polyclonal rabbit K-18 BRCA1 antibody and FITC-labeled goat anti-rabbit (green). For nucleolin staining, the cells were indirectly stained with the mouse monoclonal nucleolin antibody and FITC-labeled goat anti-mouse antibody (green). Their DNA was counterstained with PI (red), as described in Materials and Methods. The triggering threshold is depicted in red, whereas the total nuclear fluorescence contour is set on green. The LSC software allows counting of the number of fluorescent bodies (“spots”) present inside the nucleus of each individual cell. The cells were categorized based on the number of spots identified per cell and given a region number. The green # of spots histogram summarizes the number of spots per cell in a given region number. The DNA content histogram shows distribution of cells in the G1, S, and G2/M phases of the cell cycle by region number. A: Fluorescence microscopic examination of MCF7 cells revealed many cells expressed nuclear BRCA1 protein and some cells were negative (only a red nuclear signal corresponding to the PI counterstaining). Region 1 included 3.8% of cells displaying only one spot; region 2 included 0.5% cells displaying 2 spots. B: Cytometric determination of BRCA1 protein during the cell cycle. The DNA content histogram shows cells in G1, S, and G2/M cell cycle phases. BRCA1 protein immunofluorescence peaked during G1−S phase and its expression was not completely degraded during G2/M. C: Fluorescence microscopic detection of nucleolin staining in MCF7 cells showed 29.9% of cells displaying only one spot in region 1; 2.3% of cells with two spots in region 2. D: Cytometric determination of nucleolin protein during the cell cycle. The DNA content histogram shows G1, S, and G2/M cell cycle phases. The nucleolin expression peak is found during the G1−S phase of the cell cycle.
Figure 7
Confocal microscopy of γ-irradiated MCF7 cells immunostained with BRCA1 and nucleolin antibodies. A: Rabbit polyclonal BRCA1 antibody, K-18, stains multiple coarse granular foci (green) in many cell nuclei. There are some cells, which show predominantly cytoplasmic staining. B: DIC image of C. Nuclei that stain for BRCA1 (green) and nuclei that stain for nucleolin (red) are predominantly not colocalized. C: Mouse monoclonal antibody to nucleolin stains nucleoli (which are phase dense in D) and many dot-like speckles (red). D: Nomarski image of the same field as in A−C. Scale bar = 20 μm.
Figure 8
BRCA1 protein localization in HeLa cells. A: HeLa cells stained with the mouse monoclonal MS110 antibody (green) show nuclear speckles that are localized in the nucleoplasm on the periphery of nucleoli, although some are superimposed above nucleoli. B: HeLa cells stained with the rabbit polyclonal K-18 antibody (red) show nuclear speckles and nucleolar staining. C: DAPI staining of HeLa cells. D: Simultaneous MS110 and K-18 staining, counterstained with DAPI, shows colocalization (yellow) in the nucleoplasm and nucleoli. Scale bar = 10 μm. E: The rabbit polyclonal K-18 BRCA1 antibody stains many cell nuclei (green), as well as a BRCA1-staining centrosome. F: The mouse monoclonal antibody to nucleolin stains nucleoli (red). G: HeLa cells counterstained with DAPI. H: The overlay of BRCA1, nucleolin, and DAPI shows the colocalization of BRCA1 (green), merging with nucleolin (red) to show colocalization (yellow) in nucleoli and speckles in some HeLa cell nuclei In this asynchronous HeLa cell culture, however, not all of the nucleoli show BRCA1 colocalization with nucleolin; some nucleoli remain lightly stained with nucleolin, although the centrosome shows BRCA1 staining. Scale bar = 10 μm.
Figure 9
Confocal and biochemical analysis of siRNA-mediated knockdown of BRCA1 protein in HeLa cells. A: Cells transfected with plasmid pcDNA3.1 GFP show punctate nuclear staining for BRCA1 protein (green), and for nucleolin (red), which is predominantly colocalized (yellow). Original magnification ×630. B: Cells transfected with the pSUPER GFP plasmid show punctate nuclear staining, in which BRCA1 (green) staining is sometimes, but not always colocalized with nucleolin (yellow). Original magnification ×630. C: BRCA1 protein staining was diminished in cells transfected with the pSUPER HBRCA1 plasmid; counterstained with DAPI. Original magnification ×630. D: The 220-kDa BRCA1 protein is significantly knocked down in whole cell lysates from HeLa cells transfected with pSUPER HBRCA1 plasmid (lane 3); whereas the 220-kDa BRCA1 protein is clearly visible in control cells transfected with empty pSUPER plasmid (lane 1), or pSUPER GFP plasmid (lane 2). The loading control α-tubulin, shows equivalent concentrations in the lysates, and is not diminished in the siRNA-HBRCA1-treated cells.
Similar articles
- Phosphorylated BRCA1 is predominantly located in the nucleus and mitochondria.
Coene ED, Hollinshead MS, Waeytens AA, Schelfhout VR, Eechaute WP, Shaw MK, Van Oostveldt PM, Vaux DJ. Coene ED, et al. Mol Biol Cell. 2005 Feb;16(2):997-1010. doi: 10.1091/mbc.e04-10-0895. Epub 2004 Dec 9. Mol Biol Cell. 2005. PMID: 15591126 Free PMC article. - Morphometry of nucleoli and expression of nucleolin analyzed by laser scanning cytometry in mitogenically stimulated lymphocytes.
Gorczyca W, Smolewski P, Grabarek J, Ardelt B, Ita M, Melamed MR, Darzynkiewicz Z. Gorczyca W, et al. Cytometry. 2001 Nov 1;45(3):206-13. doi: 10.1002/1097-0320(20011101)45:3<206::aid-cyto1164>3.0.co;2-9. Cytometry. 2001. PMID: 11746089 - Nucleolar localization of BRCA1 protein in human breast cancer.
Tulchin N, Ornstein L, Bleiweiss IJ, Mendlowitz M, Weinstein E, Dikman SH. Tulchin N, et al. Int J Oncol. 1998 Sep;13(3):513-8. Int J Oncol. 1998. PMID: 9683786 - Identification and characterization of nucleolin as a COUP-TFII coactivator of retinoic acid receptor β transcription in breast cancer cells.
Litchfield LM, Riggs KA, Hockenberry AM, Oliver LD, Barnhart KG, Cai J, Pierce WM Jr, Ivanova MM, Bates PJ, Appana SN, Datta S, Kulesza P, McBryan J, Young LS, Klinge CM. Litchfield LM, et al. PLoS One. 2012;7(5):e38278. doi: 10.1371/journal.pone.0038278. Epub 2012 May 31. PLoS One. 2012. PMID: 22693611 Free PMC article. - The roles of nucleolin subcellular localization in cancer.
Berger CM, Gaume X, Bouvet P. Berger CM, et al. Biochimie. 2015 Jun;113:78-85. doi: 10.1016/j.biochi.2015.03.023. Epub 2015 Apr 9. Biochimie. 2015. PMID: 25866190 Review.
Cited by
- Quantitative analysis of BTF3, HINT1, NDRG1 and ODC1 protein over-expression in human prostate cancer tissue.
Symes AJ, Eilertsen M, Millar M, Nariculam J, Freeman A, Notara M, Feneley MR, Patel HR, Masters JR, Ahmed A. Symes AJ, et al. PLoS One. 2013 Dec 27;8(12):e84295. doi: 10.1371/journal.pone.0084295. eCollection 2013. PLoS One. 2013. PMID: 24386364 Free PMC article. - Genome wide proteomics of ERBB2 and EGFR and other oncogenic pathways in inflammatory breast cancer.
Zhang EY, Cristofanilli M, Robertson F, Reuben JM, Mu Z, Beavis RC, Im H, Snyder M, Hofree M, Ideker T, Omenn GS, Fanayan S, Jeong SK, Paik YK, Zhang AF, Wu SL, Hancock WS. Zhang EY, et al. J Proteome Res. 2013 Jun 7;12(6):2805-17. doi: 10.1021/pr4001527. Epub 2013 May 22. J Proteome Res. 2013. PMID: 23647160 Free PMC article. - Localization of BRCA1 protein in breast cancer tissue and cell lines with mutations.
Tulchin N, Ornstein L, Dikman S, Strauchen J, Jaffer S, Nagi C, Bleiweiss I, Kornreich R, Edelmann L, Brown K, Bodian C, Nair VD, Chambon M, Woods NT, Monteiro AN. Tulchin N, et al. Cancer Cell Int. 2013 Jul 15;13(1):70. doi: 10.1186/1475-2867-13-70. Cancer Cell Int. 2013. PMID: 23855721 Free PMC article. - Nucleolin promotes Ang II‑induced phenotypic transformation of vascular smooth muscle cells via interaction with tropoelastin mRNA.
Fang L, Zhang PF, Wang KK, Xiao ZL, Yang M, Yu ZX. Fang L, et al. Int J Mol Med. 2019 Apr;43(4):1597-1610. doi: 10.3892/ijmm.2019.4090. Epub 2019 Feb 4. Int J Mol Med. 2019. PMID: 30720050 Free PMC article. - Nucleic Acid Aptamers as Potential Therapeutic and Diagnostic Agents for Lymphoma.
Shum KT, Zhou J, Rossi JJ. Shum KT, et al. J Cancer Ther. 2013 Jun 1;4(4):872-890. doi: 10.4236/jct.2013.44099. J Cancer Ther. 2013. PMID: 25057429 Free PMC article.
References
- Newman B, Mu H, Butler LM, Millikan RC, Moorman PG, King MC. Frequency of breast cancer attributable to BRCA1 in a population based series of American women. JAMA. 1998;279:915–921. - PubMed
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal A, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal JA, Tran T, McClure M, Frye C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, Stone S, Bayer S, Christian W, Bogden R, Dayananth P, Ward J, Tonin P, Narod S, Bristow PK, Norris FH, Helvering L, Morrison P, Rosteck P, Lei M, Barrett JC, Lewis C, Neuhausen S, Cannon-Albright L, Goldgar D, Wiseman R, Kamb A, Skolnick MH. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. - PubMed
- Tavtigian SV, Simard J, Rommens J, Couch F, Shattuck-Eidens D, Neuhausen S, Merajver S, Thorlacius S, Offit K, Stoppa-Lyonnet D, Belanger C, Bell R, Berry S, Bogden R, Chen Q, Davis T, Dumont M, Frye C, Hattier T, Jammulapati S, Janecki T, Jiang P, Kehrer R, Leblanc JF, Mitchell JT, McArthur-Morrison J, Nguyen K, Peng Y, Samson C, Schroeder M, Snyder SC, Steele L, Stringfellow M, Stroup C, Swedlund B, Swense J, Teng D, Thomas A, Tran T, Tranchant M, Weaver-Feldhaus J, Wong AK, Shizuya H, Eyfjord JE, Cannon-Albright L, Tranchant M, Labrie F, Skolnick MH, Weber B, Kamb A, Goldgar DE. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996;12:333–337. - PubMed
- Merajver SD, Frank TS, Xu J, Pham T, Calzone KA, Bennett-Baker P, Chamberlain J, Boyd J, Garber JE, Collins FS, Weber BL. Germline BRCA1 mutations and loss of the wild-type allele in tumors from families with early onset breast and ovarian cancer. Clin Cancer Res. 1995;1:539–544. - PubMed
- Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Bennett LM, Haugen-Strano A, Swensen J, Miki Y, Eddington K, McClure M, Frye C, Weaver-Feldhaus J, Ding W, Gholami Z, Soderkvist P, Terry L, Jhanwar S, Berchuck A, Iglehart D, Marks J, Ballinger DG, Barrett JC, Skolnick MH, Kamb A, Wiseman R. bRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994;266:120–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA92309/CA/NCI NIH HHS/United States
- U01 CA116167/CA/NCI NIH HHS/United States
- 1R24CAO95823-01/PHS HHS/United States
- CA116167/CA/NCI NIH HHS/United States
- R01 CA116167/CA/NCI NIH HHS/United States
- R24 CA095823/CA/NCI NIH HHS/United States
- R01 CA092309/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous