A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses - PubMed (original) (raw)
A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses
Caroline E Lilley et al. EMBO J. 2010.
Abstract
The ICP0 protein of herpes simplex virus type 1 is an E3 ubiquitin ligase and transactivator required for the efficient switch between latent and lytic infection. As DNA damaging treatments are known to reactivate latent virus, we wished to explore whether ICP0 modulates the cellular response to DNA damage. We report that ICP0 prevents accumulation of repair factors at cellular damage sites, acting between recruitment of the mediator proteins Mdc1 and 53BP1. We identify RNF8 and RNF168, cellular histone ubiquitin ligases responsible for anchoring repair factors at sites of damage, as new targets for ICP0-mediated degradation. By targeting these ligases, ICP0 expression results in loss of ubiquitinated forms of H2A, mobilization of DNA repair proteins and enhanced viral fitness. Our study raises the possibility that the ICP0-mediated control of histone ubiquitination may link DNA repair, relief of transcriptional repression, and activation of latent viral genomes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
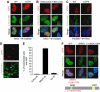
Expression of ICP0 prevents accumulation of DNA repair proteins at IRIF. (A) HeLa cells were infected with WT or ICP0-null HSV-1 at an MOI of 5 for 2 h and treated with 10 Gy IR. Cells were fixed 1 h post IR, stained for ICP4 and assessed for localization of proteins reacting with an antibody generated to phosphorylated ATM. Nuclei were stained with DAPI as shown in the merged image. (B) HeLa cells were infected with ΔUL8 or ΔUL5 mutants of HSV-1 at an MOI of 5 and then treated with 10 Gy IR. Cells were fixed 1 h post IR, and stained with antibodies to ICP0 and phosphorylated ATM. (C) ICP0 can disrupt pre-established IRIF. Uninfected HeLa cells were irradiated at 10 Gy and infected 1 h post IR with WT or ICP0-null HSV-1 at an MOI of 5 for 2 h. Cells were fixed 2 h post infection and stained with antibodies to ICP4 and phosphorylated ATM. (D) HeLa cells were transfected with an ICP0 expression plasmid for 16 h and then treated with 10 Gy IR. Cells were fixed 1 h post IR, and stained with antibodies to ICP0 and phosphorylated ATM. (E) Transfected HeLa cells (200) were scored for the localization at IRIF of proteins reacting with phosphorylated ATM antibody. Data are represented as mean±s.e.m. (F) HeLa cells were transfected with WT or RING mutant ICP0 expression plasmids for 16 h and then treated with 10 Gy IR. Cells were fixed 1 h post IR, and stained with antibodies to ICP0 and phosphorylated ATM.
Figure 2
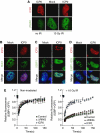
The level of the ICP0-induced block to IRIF. (A) ICP0 does not affect initial phosphorylation of H2AX. HeLa cells were transfected with an ICP0 expression plasmid for 16 h and then mock treated or treated with 10 Gy IR. Cells were fixed 15 min post IR and stained for ICP0 and γH2AX. (B) ICP0 blocks IRIF formation downstream of Mdc1. HeLa cells were transfected with an ICP0 expression plasmid for 16 h and then treated with 10 Gy IR. Cells were fixed 1 h post IR and stained for ICP0 and Mdc1. (C) ICP0 blocks IRIF upstream of 53BP1. HeLa cells were transfected with an ICP0 expression plasmid for 16 h and then treated with 10 Gy IR. Cells were fixed 1 h post IR and stained for ICP0 and 53BP1. (D) ICP0 expression does not block phosphorylation of 53BP1. HeLa cells were transfected with an ICP0 expression plasmid for 16 h and then treated with 10 Gy IR. Cells were fixed 1 h post IR and stained for ICP0 and 53BP1-S25. (E) ICP0 prevents the IR-induced decrease in 53BP1 mobility. U20S cells stably expressing GFP-tagged 53BP1 were transfected with WT or ΔRING ICP0; 16 h post transfection, cells were subject to 10 Gy IR and 15 min post IR, 53BP1 mobility was assessed through FRAP analysis. Adjusted fluorescence intensity is shown as a fraction of the pre-bleach intensity.
Figure 3
ICP0 expression induces loss of ubiquitinated forms of H2A and H2AX. (A) HeLa cells were infected with recombinant adenoviruses expressing either GFP or ICP0, plus recombinant adenovirus expressing the tet activator (1:1) at a total MOI of 50 or 10. Cells were irradiated 23 h.p.i. and then collected 1 h post IR. Lysates and acid soluble chromatin fractions prepared and analysed by immunoblotting for levels and ubiquitination status of H2A, H2AX, and γH2AX. (B) 293T cells were transfected with ICP0 and his-tagged ubiquitin. Cells were lysed in denaturing conditions 24 h post transfection, and his-ubiquitin-conjugated proteins were purified over nickel beads (Ni–NTA). Input (5%) and eluted proteins were analysed by immunoblotting for levels and ubiquitination status of H2A and H2AX. (C) 293T cells were transfected with WT or mutant versions of ICP0 and his-tagged ubiquitin. Samples were purified and blotted as in (B).
Figure 4
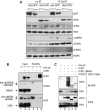
ICP0 induces degradation of RNF8 and RNF168. (A) HeLa cells were infected with WT or ΔICP0 HSV-1 at an MOI of 5 and lysates prepared for immunoblotting over a timecourse of infection. Degradation of RNF168 and loss of ubiquitinated H2A were detectable at 2 h post infection, whereas DNA-PKcs and RNF8 degradation were detectable at 4 h post infection. Levels of all degradation substrates were rescued by proteasome inhibitors added at 2 h (compare levels of RNF8 and RNF168 at 2 h to levels at 8 h in the presence of MG132 and epox). Ku70 served as a loading control. (B) HeLa cells were transfected with Flag-RNF8 or HA-RNF168 and either ICP0 WT or the ΔRING mutant in a ratio of 3:1, where ICP0 was in excess. Cells were collected at 20 h post transfection, lysates were prepared for immunoblotting and assessed for levels of RNF8 and RNF168. GAPDH was used as a loading control. (C) HeLa cells were infected with recombinant adenoviruses expressing either GFP or ICP0, plus recombinant adenovirus expressing the tet activator (1:1) at a total MOI of 50 or 10. Cells were irradiated 23 h.p.i. and then collected 1 h post IR. Lysates were analysed by immunoblotting and assessed for levels of RNF8 and RNF168. GAPDH was used as a loading control.
Figure 5
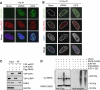
ICP0 co-localizes with, binds, and ubiquitinates RNF8. (A) HeLa cells were co-transfected with Flag-RNF8 and GFP-ICP0 or GFP-ΔRING ICP0 for 16 h (3:1 ratio of Flag-RNF8:GFP-ICP0). The co-localization of ICP0 and Flag-RNF8 was assessed by immunofluorescence. (B) HeLa cells were co-transfected with Flag-RNF8 and GFP-ICP0 or GFP-ΔRING ICP0 at the same ratio as in (A) for 16 h and then treated with 10 Gy IR. Localization of ICP0, Flag-RNF8, and 53BP1 were assessed by immunofluorescence 1 h post IR. For four-colour immunofluorescence, nuclei (as visualized by DAPI staining) are outlined in white. (C) Flag-RNF8 was co-transfected with TAP-ICP0 or TAP-mRFP into 293T cells and proteasome inhibitors were added 16 h post transfection to prevent degradation of RNF8. At 24 h post transfection, lysates were immunoprecipitated with streptavidin–sepharose beads overnight at 4°C and purified proteins were analysed by immunoblotting. Inputs (5%) and affinity purifications (AP) are shown. GAPDH was used as a loading control. (D) ICP0 ubiquitinates purified RNF8 in vitro. Ubiquitination reactions were carried out in the presence of purified Flag-RNF8 C403S and either WT, ΔRING, or RING-only (1–323) versions of ICP0. The ubiquitination status of RNF8 was analysed by immunoblotting with anti-Flag antibody (left panel). The band present in all RNF8-containing lanes is either a non-specific band or an SDS-resistant dimer of RNF8 C403S. The ability of ICP0 mutants to form poly-ubiquitin chains was assessed by immunoblotting with anti-ubiquitin antibody (right panel).
Figure 6
Rescue of the ICP0-induced block to IRIF and negative effect of RNF8 on plaque-forming efficiency of an ICP0-null virus. (A) Combined over-expression of RNF8 WT and RNF168 WT rescues the ICP0-induced block to IRIF. HeLa cells were transfected with GFP-ICP0 in the presence of Flag-RNF8 WT or C403S and Flag-RNF168 WT or C19S. The cells were exposed to 10 Gy IR at 24 h post transfection, fixed at 1 h post IR, and imaged for ICP0, Flag, and 53BP1 (three left columns), or ICP0, γH2AX, and 53BP1 (right column). For four-colour immunofluorescence, nuclei (as visualized by DAPI staining) are outlined in white. In the merged images of the cells in which 53BP1 IRIF are rescued, the Flag signal (RNF8 and RNF168) co-localizes with both ICP0 and 53BP1. (B) Transfected cells (200) from (A) were scored for presence or absence of 53BP1 at IRIF. (C) RNF8 inhibits plaque-forming efficiency of ICP0 mutant viruses. RNF8−/− MEFs or RNF8−/− MEFs reconstituted with RNF8 were infected with WT or ICP0 mutant virus. Relative probabilities of plaque formation were calculated by comparing the numbers of plaques on the different cell lines at each separate dilution of virus. The decrease in ICP0-null virus plaque-forming ability in cells expressing RNF8 ranged from 2.5- to 6-fold, with an average of 3.7-fold. The decrease in ΔRING ICP0 plaque-forming ability in cells expressing RNF8 ranged from three- to five-fold, with an average of 4.2-fold. All infections were carried out at least in duplicate and the experiment was repeated four times. Data are represented as mean±s.e.m. (D) Model for the role of ICP0 in degrading RNF8 and RNF168 to limit H2A ubiquitination and prevent the formation of IRIF. ICP0 targets both RNF8 and RNF168 for proteasome-mediated degradation. The resultant loss of ubiquitinated H2A on cellular DNA prevents the accumulation of downstream repair factors at IRIF. The potential links between ICP0, H2A ubiquitination, and transctiptional silencing are depicted.
Similar articles
- Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain.
Chaurushiya MS, Lilley CE, Aslanian A, Meisenhelder J, Scott DC, Landry S, Ticau S, Boutell C, Yates JR 3rd, Schulman BA, Hunter T, Weitzman MD. Chaurushiya MS, et al. Mol Cell. 2012 Apr 13;46(1):79-90. doi: 10.1016/j.molcel.2012.02.004. Epub 2012 Mar 7. Mol Cell. 2012. PMID: 22405594 Free PMC article. - The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0.
Lilley CE, Chaurushiya MS, Boutell C, Everett RD, Weitzman MD. Lilley CE, et al. PLoS Pathog. 2011 Jun;7(6):e1002084. doi: 10.1371/journal.ppat.1002084. Epub 2011 Jun 16. PLoS Pathog. 2011. PMID: 21698222 Free PMC article. - Ubiquitin-H2AX fusions render 53BP1 recruitment to DNA damage sites independent of RNF8 or RNF168.
Kocyłowski MK, Rey AJ, Stewart GS, Halazonetis TD. Kocyłowski MK, et al. Cell Cycle. 2015;14(11):1748-58. doi: 10.1080/15384101.2015.1010918. Cell Cycle. 2015. PMID: 25695757 Free PMC article. - Identification of a novel higher molecular weight isoform of USP7/HAUSP that interacts with the Herpes simplex virus type-1 immediate early protein ICP0.
Antrobus R, Boutell C. Antrobus R, et al. Virus Res. 2008 Oct;137(1):64-71. doi: 10.1016/j.virusres.2008.05.017. Epub 2008 Jul 17. Virus Res. 2008. PMID: 18590780 - The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection.
Rodríguez MC, Dybas JM, Hughes J, Weitzman MD, Boutell C. Rodríguez MC, et al. Virus Res. 2020 Aug;285:198015. doi: 10.1016/j.virusres.2020.198015. Epub 2020 May 13. Virus Res. 2020. PMID: 32416261 Free PMC article. Review.
Cited by
- Ubiquitination at the interface of tumor viruses and DNA damage responses.
Dybas JM, Herrmann C, Weitzman MD. Dybas JM, et al. Curr Opin Virol. 2018 Oct;32:40-47. doi: 10.1016/j.coviro.2018.08.017. Epub 2018 Sep 24. Curr Opin Virol. 2018. PMID: 30261451 Free PMC article. Review. - DNA virus replication compartments.
Schmid M, Speiseder T, Dobner T, Gonzalez RA. Schmid M, et al. J Virol. 2014 Feb;88(3):1404-20. doi: 10.1128/JVI.02046-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257611 Free PMC article. Review. - Ubiquitin Ligases Involved in the Regulation of Wnt, TGF-β, and Notch Signaling Pathways and Their Roles in Mouse Development and Homeostasis.
Baloghova N, Lidak T, Cermak L. Baloghova N, et al. Genes (Basel). 2019 Oct 16;10(10):815. doi: 10.3390/genes10100815. Genes (Basel). 2019. PMID: 31623112 Free PMC article. Review. - Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication.
Adeyemi RO, Landry S, Davis ME, Weitzman MD, Pintel DJ. Adeyemi RO, et al. PLoS Pathog. 2010 Oct 7;6(10):e1001141. doi: 10.1371/journal.ppat.1001141. PLoS Pathog. 2010. PMID: 20949077 Free PMC article. - Viral Modulation of the DNA Damage Response and Innate Immunity: Two Sides of the Same Coin.
Lopez A, Nichols Doyle R, Sandoval C, Nisson K, Yang V, Fregoso OI. Lopez A, et al. J Mol Biol. 2022 Mar 30;434(6):167327. doi: 10.1016/j.jmb.2021.167327. Epub 2021 Oct 22. J Mol Biol. 2022. PMID: 34695379 Free PMC article. Review.
References
- Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421: 499–506 - PubMed
- Botvinick EL, Berns MW (2005) Internet-based robotic laser scissors and tweezers microscopy. Microsc Res Tech 68: 65–74 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA097093/CA/NCI NIH HHS/United States
- MC_UP_A550_1030/MRC_/Medical Research Council/United Kingdom
- CA97093/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- MC_U130169966/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous